Varenicline is a potent partial agonist at α6β2* nicotinic acetylcholine receptors in rat and monkey striatum
- PMID: 22550286
- PMCID: PMC3400806
- DOI: 10.1124/jpet.112.194852
Varenicline is a potent partial agonist at α6β2* nicotinic acetylcholine receptors in rat and monkey striatum
Abstract
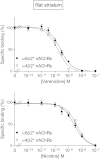
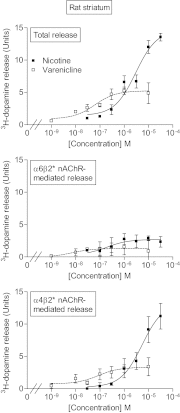
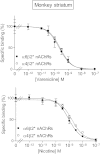
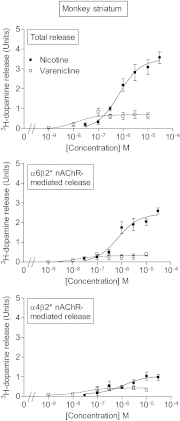
Extensive evidence indicates that varenicline reduces nicotine craving and withdrawal symptoms by modulating dopaminergic function at α4β2* nicotinic acetylcholine receptors (nAChRs) (the asterisk indicates the possible presence of other nicotinic subunits in the receptor complex). More recent data suggest that α6β2* nAChRs also regulate dopamine release and mediate nicotine reinforcement. The present experiments were therefore done to test the effect of varenicline on α6β2* nAChRs and their function, because its interaction with this subtype is currently unclear. Receptor competition studies showed that varenicline inhibited α6β2* nAChR binding (K(i) = 0.12 nM) as potently as α4β2* nAChR binding (K(i) = 0.14 nM) in rat striatal sections and with ∼20-fold greater affinity than nicotine. Functionally, varenicline was more potent in stimulating α6β2* versus α4β2* nAChR-mediated [(3)H]dopamine release from rat striatal synaptosomes with EC(50) values of 0.007 and 0.086 μM, respectively. However, it acted as a partial agonist on α6β2* and α4β2* nAChR-mediated [(3)H]dopamine release with maximal efficacies of 49 and 24%, respectively, compared with nicotine. We also evaluated varenicline's action in striatum of monkeys, a useful animal model for comparison with humans. Varenicline again potently inhibited monkey striatal α6β2* (K(i) = 0.13 nM) and α4β2* (K(i) = 0.19 nM) nAChRs in competition studies. Functionally, it potently stimulated both α6β2* (EC(50) = 0.014 μM) and α4β2* (EC(50) = 0.029 μM) nAChR-mediated [(3)H]dopamine release from monkey striatal synaptosomes, again acting as a partial agonist relative to nicotine at both subtypes. These data suggest that the ability of varenicline to interact at α6β2* nAChRs may contribute to its efficacy as a smoking cessation aid.
Figures
Similar articles
-
The contribution of agonist and antagonist activities of α4β2* nAChR ligands to smoking cessation efficacy: a quantitative analysis of literature data.Psychopharmacology (Berl). 2018 Sep;235(9):2479-2505. doi: 10.1007/s00213-018-4921-9. Epub 2018 Jul 7. Psychopharmacology (Berl). 2018. PMID: 29980822 Review.
-
UB-165: a novel nicotinic agonist with subtype selectivity implicates the alpha4beta2* subtype in the modulation of dopamine release from rat striatal synaptosomes.J Neurosci. 2000 Apr 15;20(8):2783-91. doi: 10.1523/JNEUROSCI.20-08-02783.2000. J Neurosci. 2000. PMID: 10751429 Free PMC article.
-
Pharmacological profile of the alpha4beta2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid.Neuropharmacology. 2007 Mar;52(3):985-94. doi: 10.1016/j.neuropharm.2006.10.016. Epub 2006 Dec 8. Neuropharmacology. 2007. PMID: 17157884
-
α6β2*-subtype nicotinic acetylcholine receptors are more sensitive than α4β2*-subtype receptors to regulation by chronic nicotine administration.J Neurochem. 2014 Jul;130(2):185-98. doi: 10.1111/jnc.12721. Epub 2014 Apr 19. J Neurochem. 2014. PMID: 24661093 Free PMC article.
-
Diverse strategies targeting α7 homomeric and α6β2* heteromeric nicotinic acetylcholine receptors for smoking cessation.Ann N Y Acad Sci. 2014 Oct;1327(1):27-45. doi: 10.1111/nyas.12421. Epub 2014 Apr 14. Ann N Y Acad Sci. 2014. PMID: 24730978 Free PMC article. Review.
Cited by
-
Neuronal nicotinic acetylcholine receptors: common molecular substrates of nicotine and alcohol dependence.Front Psychiatry. 2013 Apr 30;4:29. doi: 10.3389/fpsyt.2013.00029. eCollection 2013. Front Psychiatry. 2013. PMID: 23641218 Free PMC article.
-
Therapeutic concentrations of varenicline in the presence of nicotine increase action potential firing in human adrenal chromaffin cells.J Neurochem. 2017 Jan;140(1):37-52. doi: 10.1111/jnc.13883. Epub 2016 Dec 9. J Neurochem. 2017. PMID: 27805736 Free PMC article.
-
Recent Advances in the Pharmacology of Tardive Dyskinesia.Clin Psychopharmacol Neurosci. 2020 Nov 30;18(4):493-506. doi: 10.9758/cpn.2020.18.4.493. Clin Psychopharmacol Neurosci. 2020. PMID: 33124584 Free PMC article. Review.
-
Effect of Varenicline on Tardive Dyskinesia: A Pilot Study.Clin Psychopharmacol Neurosci. 2021 May 31;19(2):355-360. doi: 10.9758/cpn.2021.19.2.355. Clin Psychopharmacol Neurosci. 2021. PMID: 33888664 Free PMC article.
-
A behavioral economic analysis of the value-enhancing effects of nicotine and varenicline and the role of nicotinic acetylcholine receptors in male and female rats.Behav Pharmacol. 2018 Sep;29(6):493-502. doi: 10.1097/FBP.0000000000000404. Behav Pharmacol. 2018. PMID: 29634495 Free PMC article.
References
-
- Anderson DJ, Malysz J, Grønlien JH, El Kouhen R, Håkerud M, Wetterstrand C, Briggs CA, Gopalakrishnan M. (2009) Stimulation of dopamine release by nicotinic acetylcholine receptor ligands in rat brain slices correlates with the profile of high, but not low, sensitivity α4β2 subunit combination. Biochem Pharmacol 78:844–851 - PubMed
-
- Artymyshyn R, Smith A, Wolfe BB. (1990) The use of 3H standards in 125I autoradiography. J Neurosci Methods 32:185–192 - PubMed
-
- Barik J, Wonnacott S. (2009) Molecular and cellular mechanisms of action of nicotine in the CNS. Handb Exp Pharmacol 192:173–207 - PubMed
-
- Biala G, Staniak N, Budzynska B. (2010) Effects of varenicline and mecamylamine on the acquisition, expression, and reinstatement of nicotine-conditioned place preference by drug priming in rats. Naunyn Schmiedebergs Arch Pharmacol 381:361–370 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

