The ST2 pathway is involved in acute pancreatitis: a translational study in humans and mice
- PMID: 22542450
- PMCID: PMC3366073
- DOI: 10.1016/j.ajpath.2012.03.009
The ST2 pathway is involved in acute pancreatitis: a translational study in humans and mice
Abstract
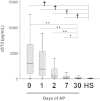
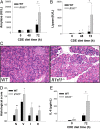
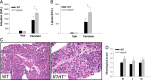
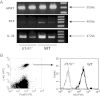
Acute pancreatitis (AP) is an inflammatory disease in which the regulatory pathways are not clearly elucidated. Activation of interleukin 1β (IL-1β) and immunomodulation via MyD88, the first signaling molecule in the ST2 pathway, seem to be involved. Because IL-33, the ST2 ligand, is an IL-1 family member and acts as an alarmin, we explored the ST2 pathway in human and mouse AP. Soluble ST2 was assayed by enzyme-linked immunosorbent assay (ELISA) in plasma of 44 patients admitted for AP. The levels of soluble ST2 increased early during AP and correlated with parameters of severity. Under two different experimental models of AP (ie, choline-deficient-ethionine-supplemented diet and cerulein injections), ST2-deficient mice (Il1rl1(-/-)) presented with more severe disease than wild-type mice, with increased activation of mast cells. In vitro, Il1rl1(-/-) bone-marrow-derived mast cells exhibited exacerbated degranulation, compared with the wild type. Flow cytometry identified mast cells as the main peritoneal population expressing ST2. Using immunohistochemistry and ELISA, we showed constitutive expression of IL-33 in murine pancreas and its release during experimental AP. Correlated with AP severity, increased soluble ST2 levels evoke involvement of the ST2 pathway in human AP. Furthermore, our experimental data suggest a protective role for ST2 during AP, highlighting the potential regulatory role of mast cells and the possibility of the ST2 pathway as a new therapeutic target in AP.
Copyright © 2012 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures

Similar articles
-
The IL-33/ST2 pathway controls coxsackievirus B5-induced experimental pancreatitis.J Immunol. 2013 Jul 1;191(1):283-92. doi: 10.4049/jimmunol.1202806. Epub 2013 Jun 3. J Immunol. 2013. PMID: 23733876
-
IL-33 exacerbates autoantibody-induced arthritis.J Immunol. 2010 Mar 1;184(5):2620-6. doi: 10.4049/jimmunol.0902685. Epub 2010 Feb 5. J Immunol. 2010. PMID: 20139274
-
The IL-1 receptor accessory protein (AcP) is required for IL-33 signaling and soluble AcP enhances the ability of soluble ST2 to inhibit IL-33.Cytokine. 2008 Jun;42(3):358-64. doi: 10.1016/j.cyto.2008.03.008. Epub 2008 May 2. Cytokine. 2008. PMID: 18450470
-
IL-33/ST2 axis in inflammation and immunopathology.Immunol Res. 2012 Apr;52(1-2):89-99. doi: 10.1007/s12026-012-8283-9. Immunol Res. 2012. PMID: 22392053 Review.
-
Crucial and diverse role of the interleukin-33/ST2 axis in infectious diseases.Infect Immun. 2015 May;83(5):1738-48. doi: 10.1128/IAI.02908-14. Epub 2015 Feb 23. Infect Immun. 2015. PMID: 25712928 Free PMC article. Review.
Cited by
-
Circulating Markers of Necroptosis in Acute Pancreatitis.Dig Dis Sci. 2024 Sep;69(9):3333-3343. doi: 10.1007/s10620-024-08530-6. Epub 2024 Jun 28. Dig Dis Sci. 2024. PMID: 38940973 Free PMC article.
-
Novel Approach for Pancreas Transcriptomics Reveals the Cellular Landscape in Homeostasis and Acute Pancreatitis.Gastroenterology. 2024 Jun;166(6):1100-1113. doi: 10.1053/j.gastro.2024.01.043. Epub 2024 Feb 6. Gastroenterology. 2024. PMID: 38325760
-
The trigger for pancreatic disease: NLRP3 inflammasome.Cell Death Discov. 2023 Jul 14;9(1):246. doi: 10.1038/s41420-023-01550-7. Cell Death Discov. 2023. PMID: 37452057 Free PMC article. Review.
-
Serum soluble suppression of tumorigenicity 2 as a novel inflammatory marker predicts the severity of acute pancreatitis.World J Gastroenterol. 2021 Oct 14;27(38):6489-6500. doi: 10.3748/wjg.v27.i38.6489. World J Gastroenterol. 2021. PMID: 34720537 Free PMC article.
-
Electrochemical Immunosensing of ST2: A Checkpoint Target in Cancer Diseases.Biosensors (Basel). 2021 Jun 21;11(6):202. doi: 10.3390/bios11060202. Biosensors (Basel). 2021. PMID: 34205541 Free PMC article.
References
-
- Pandol S.J., Saluja A.K., Imrie C.W., Banks P.A. Acute pancreatitis: bench to bedside [Erratum appeared in Gastroenterology 2007, 133:1056] Gastroenterology. 2007;132:1127–1151. - PubMed
-
- Whitcomb D.C. Clinical practice: acute pancreatitis. N Engl J Med. 2006;354:2142–2150. - PubMed
-
- Fink G., Yang J., Carter G., Norman J. Acute pancreatitis-induced enzyme release and necrosis are attenuated by IL-1 antagonism through an indirect mechanism. J Surg Res. 1997;67:94–97. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

