DICER1 loss and Alu RNA induce age-related macular degeneration via the NLRP3 inflammasome and MyD88
- PMID: 22541070
- PMCID: PMC3351582
- DOI: 10.1016/j.cell.2012.03.036
DICER1 loss and Alu RNA induce age-related macular degeneration via the NLRP3 inflammasome and MyD88
Abstract
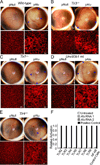
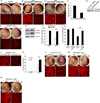
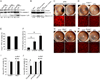
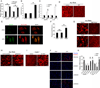
Alu RNA accumulation due to DICER1 deficiency in the retinal pigmented epithelium (RPE) is implicated in geographic atrophy (GA), an advanced form of age-related macular degeneration that causes blindness in millions of individuals. The mechanism of Alu RNA-induced cytotoxicity is unknown. Here we show that DICER1 deficit or Alu RNA exposure activates the NLRP3 inflammasome and triggers TLR-independent MyD88 signaling via IL18 in the RPE. Genetic or pharmacological inhibition of inflammasome components (NLRP3, Pycard, Caspase-1), MyD88, or IL18 prevents RPE degeneration induced by DICER1 loss or Alu RNA exposure. These findings, coupled with our observation that human GA RPE contains elevated amounts of NLRP3, PYCARD, and IL18 and evidence of increased Caspase-1 and MyD88 activation, provide a rationale for targeting this pathway in GA. Our findings also reveal a function of the inflammasome outside the immune system and an immunomodulatory action of mobile elements.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures

Similar articles
-
TLR-independent and P2X7-dependent signaling mediate Alu RNA-induced NLRP3 inflammasome activation in geographic atrophy.Invest Ophthalmol Vis Sci. 2013 Nov 11;54(12):7395-401. doi: 10.1167/iovs.13-12500. Invest Ophthalmol Vis Sci. 2013. PMID: 24114535 Free PMC article.
-
Iron Toxicity in the Retina Requires Alu RNA and the NLRP3 Inflammasome.Cell Rep. 2015 Jun 23;11(11):1686-93. doi: 10.1016/j.celrep.2015.05.023. Epub 2015 Jun 11. Cell Rep. 2015. PMID: 26074074 Free PMC article.
-
An emerging role of Alu RNA in geographic atrophy pathogenesis: the implication for novel therapeutic strategies.Discov Med. 2016 Dec;22(123):337-349. Discov Med. 2016. PMID: 28147216 Review.
-
DICER1 deficit induces Alu RNA toxicity in age-related macular degeneration.Nature. 2011 Mar 17;471(7338):325-30. doi: 10.1038/nature09830. Epub 2011 Feb 6. Nature. 2011. PMID: 21297615 Free PMC article.
-
DICER1 in the Pathogenesis of Age-related Macular Degeneration (AMD) - Alu RNA Accumulation versus miRNA Dysregulation.Aging Dis. 2020 Jul 23;11(4):851-862. doi: 10.14336/AD.2019.0809. eCollection 2020 Jul. Aging Dis. 2020. PMID: 32765950 Free PMC article. Review.
Cited by
-
Pluripotent Stem Cells in Clinical Cell Transplantation: Focusing on Induced Pluripotent Stem Cell-Derived RPE Cell Therapy in Age-Related Macular Degeneration.Int J Mol Sci. 2022 Nov 9;23(22):13794. doi: 10.3390/ijms232213794. Int J Mol Sci. 2022. PMID: 36430270 Free PMC article. Review.
-
Geographic atrophy: pathophysiology and current therapeutic strategies.Front Ophthalmol (Lausanne). 2023 Dec 5;3:1327883. doi: 10.3389/fopht.2023.1327883. eCollection 2023. Front Ophthalmol (Lausanne). 2023. PMID: 38983017 Free PMC article. Review.
-
Nanotechnology in corneal neovascularization therapy--a review.J Ocul Pharmacol Ther. 2013 Mar;29(2):124-34. doi: 10.1089/jop.2012.0158. Epub 2013 Feb 20. J Ocul Pharmacol Ther. 2013. PMID: 23425431 Free PMC article. Review.
-
Production of small RNAs by mammalian Dicer.Pflugers Arch. 2016 Jun;468(6):1089-102. doi: 10.1007/s00424-016-1817-6. Epub 2016 Apr 6. Pflugers Arch. 2016. PMID: 27048428 Free PMC article. Review.
-
Caveolin-1 Promotes Cellular Senescence in Exchange for Blocking Subretinal Fibrosis in Age-Related Macular Degeneration.Invest Ophthalmol Vis Sci. 2020 Sep 1;61(11):21. doi: 10.1167/iovs.61.11.21. Invest Ophthalmol Vis Sci. 2020. PMID: 32926104 Free PMC article.
References
-
- Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–150. - PubMed
-
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. - PubMed
-
- Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. - PubMed
-
- Ambati J, Ambati BK, Yoo SH, Ianchulev S, Adamis AP. Age-related macular degeneration: etiology, pathogenesis, and therapeutic strategies. Surv Ophthalmol. 2003;48:257–293. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- RC1EY020442/EY/NEI NIH HHS/United States
- R01 EY017182/EY/NEI NIH HHS/United States
- RC1 EY020442/EY/NEI NIH HHS/United States
- R01 EY020672/EY/NEI NIH HHS/United States
- P30 EY003040/EY/NEI NIH HHS/United States
- T32 HL091812/HL/NHLBI NIH HHS/United States
- T32HL091812/HL/NHLBI NIH HHS/United States
- R01 AI063331/AI/NIAID NIH HHS/United States
- P30 EY021721/EY/NEI NIH HHS/United States
- R01 EY022238/EY/NEI NIH HHS/United States
- UL1 TR000117/TR/NCATS NIH HHS/United States
- R01EY001545/EY/NEI NIH HHS/United States
- R01EY020672/EY/NEI NIH HHS/United States
- R01EY017950/EY/NEI NIH HHS/United States
- UL1RR033173/RR/NCRR NIH HHS/United States
- P30EY021721/EY/NEI NIH HHS/United States
- R01GM068414/GM/NIGMS NIH HHS/United States
- TL1 RR033172/RR/NCRR NIH HHS/United States
- R01EY022238/EY/NEI NIH HHS/United States
- R01 EY018350/EY/NEI NIH HHS/United States
- R01 GM068414/GM/NIGMS NIH HHS/United States
- P30EY003040/EY/NEI NIH HHS/United States
- TL1 TR000115/TR/NCATS NIH HHS/United States
- UL1 RR033173/RR/NCRR NIH HHS/United States
- R21EY019778/EY/NEI NIH HHS/United States
- R01AI063331/AI/NIAID NIH HHS/United States
- R21 EY019778/EY/NEI NIH HHS/United States
- R01 AR052756/AR/NIAMS NIH HHS/United States
- R01EY018350/EY/NEI NIH HHS/United States
- R01 EY018836/EY/NEI NIH HHS/United States
- R01 EY001545/EY/NEI NIH HHS/United States
- MC_U105663142/MRC_/Medical Research Council/United Kingdom
- K08EY021521/EY/NEI NIH HHS/United States
- K08EY021757/EY/NEI NIH HHS/United States
- R01EY017182/EY/NEI NIH HHS/United States
- K08 EY021757/EY/NEI NIH HHS/United States
- R01 EY017950/EY/NEI NIH HHS/United States
- R01AR052756/AR/NIAMS NIH HHS/United States
- UL1 TR001998/TR/NCATS NIH HHS/United States
- K08 EY021521/EY/NEI NIH HHS/United States
- R01EY018836/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

