Synaptotagmin-2 is a reliable marker for parvalbumin positive inhibitory boutons in the mouse visual cortex
- PMID: 22539967
- PMCID: PMC3335159
- DOI: 10.1371/journal.pone.0035323
Synaptotagmin-2 is a reliable marker for parvalbumin positive inhibitory boutons in the mouse visual cortex
Erratum in
- PLoS One. 2012;7(7): doi/10.1371/annotation/1c5484e5-41c0-44dc-8422-2dbd3a002f3b
Abstract
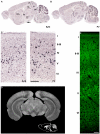
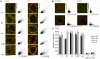
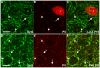
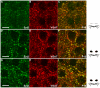
Background: Inhibitory innervation by parvalbumin (PV) expressing interneurons has been implicated in the onset of the sensitive period of visual plasticity. Immunohistochemical analysis of the development and plasticity of these inhibitory inputs is difficult because PV expression is low in young animals and strongly influenced by neuronal activity. Moreover, the synaptic boutons that PV neurons form onto each other cannot be distinguished from the innervated cell bodies by immunostaining for this protein because it is present throughout the cells. These problems call for the availability of a synaptic, activity-independent marker for PV+ inhibitory boutons that is expressed before sensitive period onset. We investigated whether synaptotagmin-2 (Syt2) fulfills these properties in the visual cortex. Syt2 is a synaptic vesicle protein involved in fast Ca(2+) dependent neurotransmitter release. Its mRNA expression follows a pattern similar to that of PV throughout the brain and is present in 30-40% of hippocampal PV expressing basket cells. Up to now, no quantitative analyses of Syt2 expression in the visual cortex have been carried out.
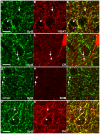
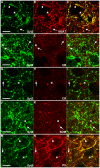
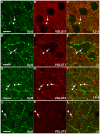
Methodology/principal findings: We used immunohistochemistry to analyze colocalization of Syt2 with multiple interneuron markers including vesicular GABA transporter VGAT, calbindin, calretinin, somatostatin and PV in the primary visual cortex of mice during development and after dark-rearing.
Conclusions/significance: We show that in the adult visual cortex Syt2 is only found in inhibitory, VGAT positive boutons. Practically all Syt2 positive boutons also contain PV and vice versa. During development, Syt2 expression can be detected in synaptic boutons prior to PV and in contrast to PV expression, Syt2 is not down-regulated by dark-rearing. These properties of Syt2 make it an excellent marker for analyzing the development and plasticity of perisomatic inhibitory innervations onto both excitatory and inhibitory neurons in the visual cortex.
Conflict of interest statement
Figures

Similar articles
-
Proportional loss of parvalbumin-immunoreactive synaptic boutons and granule cells from the hippocampus of sea lions with temporal lobe epilepsy.J Comp Neurol. 2019 Oct 1;527(14):2341-2355. doi: 10.1002/cne.24680. Epub 2019 Mar 22. J Comp Neurol. 2019. PMID: 30861128 Free PMC article.
-
Synaptotagmin2 (Syt2) Drives Fast Release Redundantly with Syt1 at the Output Synapses of Parvalbumin-Expressing Inhibitory Neurons.J Neurosci. 2017 Apr 26;37(17):4604-4617. doi: 10.1523/JNEUROSCI.3736-16.2017. Epub 2017 Mar 31. J Neurosci. 2017. PMID: 28363983 Free PMC article.
-
Pharmacological and optical activation of TrkB in Parvalbumin interneurons regulate intrinsic states to orchestrate cortical plasticity.Mol Psychiatry. 2021 Dec;26(12):7247-7256. doi: 10.1038/s41380-021-01211-0. Epub 2021 Jul 28. Mol Psychiatry. 2021. PMID: 34321594 Free PMC article.
-
Postnatal development of calcium-binding proteins immunoreactivity (parvalbumin, calbindin, calretinin) in the human entorhinal cortex.J Chem Neuroanat. 2003 Dec;26(4):311-6. doi: 10.1016/j.jchemneu.2003.09.005. J Chem Neuroanat. 2003. PMID: 14729133 Review.
-
'New' functions for 'old' proteins: the role of the calcium-binding proteins calbindin D-28k, calretinin and parvalbumin, in cerebellar physiology. Studies with knockout mice.Cerebellum. 2002 Dec;1(4):241-58. doi: 10.1080/147342202320883551. Cerebellum. 2002. PMID: 12879963 Review.
Cited by
-
Intracellular spatial transcriptomic analysis toolkit (InSTAnT).Nat Commun. 2024 Sep 6;15(1):7794. doi: 10.1038/s41467-024-49457-w. Nat Commun. 2024. PMID: 39242579 Free PMC article.
-
Clock genes control cortical critical period timing.Neuron. 2015 Apr 8;86(1):264-75. doi: 10.1016/j.neuron.2015.02.036. Epub 2015 Mar 19. Neuron. 2015. PMID: 25801703 Free PMC article.
-
Structural maturation of cortical perineuronal nets and their perforating synapses revealed by superresolution imaging.Proc Natl Acad Sci U S A. 2019 Apr 2;116(14):7071-7076. doi: 10.1073/pnas.1817222116. Epub 2019 Mar 19. Proc Natl Acad Sci U S A. 2019. PMID: 30890637 Free PMC article.
-
Inositol polyphosphate multikinase deficiency leads to aberrant induction of synaptotagmin-2 in the forebrain.Mol Brain. 2019 Jun 20;12(1):58. doi: 10.1186/s13041-019-0480-1. Mol Brain. 2019. PMID: 31221192 Free PMC article.
-
Deletion of TrkB in parvalbumin interneurons alters cortical neural dynamics.J Cell Physiol. 2022 Jan;237(1):949-964. doi: 10.1002/jcp.30571. Epub 2021 Sep 7. J Cell Physiol. 2022. PMID: 34491578 Free PMC article.
References
-
- Fagiolini M, Hensch TK. Inhibitory threshold for critical-period activation in primary visual cortex. Nature. 2000;404(6774):183–6. - PubMed
-
- Sugiyama S, Di Nardo AA, Aizawa S, Matsuo I, Volovitch M, et al. Experience-dependent transfer of Otx2 homeoprotein into the visual cortex activates postnatal plasticity. Cell. 2008;134(3):508–20. - PubMed
-
- Kawaguchi Y, Kubota Y. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb Cortex. 1997;7(6):476–86. - PubMed
-
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, et al. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5(10):793–807. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

