The Ras-like protein R-Ras2/TC21 is important for proper mammary gland development
- PMID: 22535521
- PMCID: PMC3374755
- DOI: 10.1091/mbc.E12-01-0060
The Ras-like protein R-Ras2/TC21 is important for proper mammary gland development
Abstract
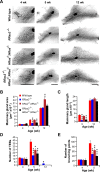
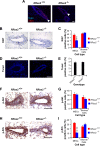
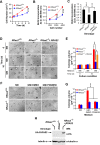
R-Ras2/TC21 is a GTPase with high sequence and signaling similarity with Ras subfamily members. Although it has been extensively studied using overexpression studies in cell lines, its physiological role remains poorly characterized. Here we used RRas2-knockout mice expressing β-galactosidase under the regulation of the endogenous RRas2 promoter to investigate the function of this GTPase in vivo. Despite its expression in tissues critical for organismal viability, RRas2(-/-) mice show no major alterations in viability, growth rates, cardiovascular parameters, or fertility. By contrast, they display a marked and specific defect in the development of the mammary gland during puberty. In the absence of R-Ras2/TC21, this gland forms reduced numbers of terminal end buds (TEBs) and ductal branches, leading to a temporal delay in the extension and arborization of the gland tree in mammary fat pads. This phenotype is linked to cell-autonomous proliferative defects of epithelial cells present in TEBs. These cells also show reduced Erk activation but wild type-like levels of phosphorylated Akt. Using compound RRas2-, HRas-, and NRas-knockout mice, we demonstrate that these GTPases act in a nonsynergistic and nonadditive manner during this morphogenic process.
Figures

Similar articles
-
Role of TC21/R-Ras2 in enhanced migration of neurofibromin-deficient Schwann cells.Oncogene. 2004 Jan 15;23(2):368-78. doi: 10.1038/sj.onc.1207075. Oncogene. 2004. PMID: 14724565 Free PMC article.
-
CUZD1 is a critical mediator of the JAK/STAT5 signaling pathway that controls mammary gland development during pregnancy.PLoS Genet. 2017 Mar 9;13(3):e1006654. doi: 10.1371/journal.pgen.1006654. eCollection 2017 Mar. PLoS Genet. 2017. PMID: 28278176 Free PMC article.
-
Constitutive overexpression of Id-1 in mammary glands of transgenic mice results in precocious and increased formation of terminal end buds, enhanced alveologenesis, delayed involution.J Cell Physiol. 2011 May;226(5):1340-52. doi: 10.1002/jcp.22462. J Cell Physiol. 2011. PMID: 20945346
-
Characterization of mutant versions of the R-RAS2/TC21 GTPase found in tumors.Oncogene. 2023 Jan;42(5):389-405. doi: 10.1038/s41388-022-02563-9. Epub 2022 Dec 7. Oncogene. 2023. PMID: 36476833 Free PMC article.
-
Cooperation of signalling pathways in embryonic mammary gland development.Nat Rev Genet. 2007 Dec;8(12):963-72. doi: 10.1038/nrg2227. Nat Rev Genet. 2007. PMID: 18007652 Review.
Cited by
-
The ancestral type of the R-RAS protein has oncogenic potential.Cell Mol Biol Lett. 2024 Feb 21;29(1):27. doi: 10.1186/s11658-024-00546-0. Cell Mol Biol Lett. 2024. PMID: 38383288 Free PMC article.
-
Activating Mutations of RRAS2 Are a Rare Cause of Noonan Syndrome.Am J Hum Genet. 2019 Jun 6;104(6):1223-1232. doi: 10.1016/j.ajhg.2019.04.013. Epub 2019 May 23. Am J Hum Genet. 2019. PMID: 31130282 Free PMC article.
-
Sympathetic nerve signals: orchestrators of mammary development and stem cell vitality.J Mol Cell Biol. 2024 Oct 21;16(5):mjae020. doi: 10.1093/jmcb/mjae020. J Mol Cell Biol. 2024. PMID: 38740522 Free PMC article.
-
Selective Recognition of H3.1K36 Dimethylation/H4K16 Acetylation Facilitates the Regulation of All-trans-retinoic Acid (ATRA)-responsive Genes by Putative Chromatin Reader ZMYND8.J Biol Chem. 2016 Feb 5;291(6):2664-81. doi: 10.1074/jbc.M115.679985. Epub 2015 Dec 11. J Biol Chem. 2016. PMID: 26655721 Free PMC article.
-
The Syk Kinase Promotes Mammary Epithelial Integrity and Inhibits Breast Cancer Invasion by Stabilizing the E-Cadherin/Catenin Complex.Cancers (Basel). 2019 Dec 7;11(12):1974. doi: 10.3390/cancers11121974. Cancers (Basel). 2019. PMID: 31817924 Free PMC article.
References
-
- Arora S, Matta A, Shukla NK, Deo SV, Ralhan R. Identification of differentially expressed genes in oral squamous cell carcinoma. Mol Carcinog. 2005;42:97–108. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

