Plant hormone jasmonate prioritizes defense over growth by interfering with gibberellin signaling cascade
- PMID: 22529386
- PMCID: PMC3358897
- DOI: 10.1073/pnas.1201616109
Plant hormone jasmonate prioritizes defense over growth by interfering with gibberellin signaling cascade
Abstract
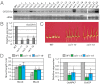
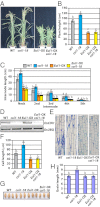
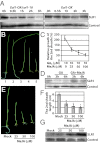
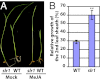
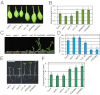
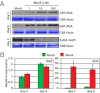
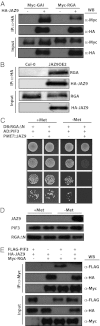
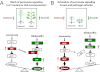
Plants must effectively defend against biotic and abiotic stresses to survive in nature. However, this defense is costly and is often accompanied by significant growth inhibition. How plants coordinate the fluctuating growth-defense dynamics is not well understood and remains a fundamental question. Jasmonate (JA) and gibberellic acid (GA) are important plant hormones that mediate defense and growth, respectively. Binding of bioactive JA or GA ligands to cognate receptors leads to proteasome-dependent degradation of specific transcriptional repressors (the JAZ or DELLA family of proteins), which, at the resting state, represses cognate transcription factors involved in defense (e.g., MYCs) or growth [e.g. phytochrome interacting factors (PIFs)]. In this study, we found that the coi1 JA receptor mutants of rice (a domesticated monocot crop) and Arabidopsis (a model dicot plant) both exhibit hallmark phenotypes of GA-hypersensitive mutants. JA delays GA-mediated DELLA protein degradation, and the della mutant is less sensitive to JA for growth inhibition. Overexpression of a selected group of JAZ repressors in Arabidopsis plants partially phenocopies GA-associated phenotypes of the coi1 mutant, and JAZ9 inhibits RGA (a DELLA protein) interaction with transcription factor PIF3. Importantly, the pif quadruple (pifq) mutant no longer responds to JA-induced growth inhibition, and overexpression of PIF3 could partially overcome JA-induced growth inhibition. Thus, a molecular cascade involving the COI1-JAZ-DELLA-PIF signaling module, by which angiosperm plants prioritize JA-mediated defense over growth, has been elucidated.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
The Arabidopsis DELLA RGA-LIKE3 is a direct target of MYC2 and modulates jasmonate signaling responses.Plant Cell. 2012 Aug;24(8):3307-19. doi: 10.1105/tpc.112.101428. Epub 2012 Aug 14. Plant Cell. 2012. PMID: 22892320 Free PMC article.
-
A bHLH-type transcription factor, ABA-INDUCIBLE BHLH-TYPE TRANSCRIPTION FACTOR/JA-ASSOCIATED MYC2-LIKE1, acts as a repressor to negatively regulate jasmonate signaling in arabidopsis.Plant Cell. 2013 May;25(5):1641-56. doi: 10.1105/tpc.113.111112. Epub 2013 May 14. Plant Cell. 2013. PMID: 23673982 Free PMC article.
-
A critical role of two positively charged amino acids in the Jas motif of Arabidopsis JAZ proteins in mediating coronatine- and jasmonoyl isoleucine-dependent interactions with the COI1 F-box protein.Plant J. 2008 Sep;55(6):979-88. doi: 10.1111/j.1365-313X.2008.03566.x. Epub 2008 Jun 10. Plant J. 2008. PMID: 18547396 Free PMC article.
-
JAZ repressors and the orchestration of phytohormone crosstalk.Trends Plant Sci. 2012 Jan;17(1):22-31. doi: 10.1016/j.tplants.2011.10.006. Epub 2011 Nov 21. Trends Plant Sci. 2012. PMID: 22112386 Review.
-
Plant oxylipins: COI1/JAZs/MYC2 as the core jasmonic acid-signalling module.FEBS J. 2009 Sep;276(17):4682-92. doi: 10.1111/j.1742-4658.2009.07194.x. Epub 2009 Aug 3. FEBS J. 2009. PMID: 19663905 Review.
Cited by
-
A fungal endophyte helps plants to tolerate root herbivory through changes in gibberellin and jasmonate signaling.New Phytol. 2016 Aug;211(3):1065-76. doi: 10.1111/nph.13957. Epub 2016 Apr 6. New Phytol. 2016. PMID: 27061745 Free PMC article.
-
Jasmonic Acid Signaling and Molecular Crosstalk with Other Phytohormones.Int J Mol Sci. 2021 Mar 13;22(6):2914. doi: 10.3390/ijms22062914. Int J Mol Sci. 2021. PMID: 33805647 Free PMC article. Review.
-
Diuretics prime plant immunity in Arabidopsis thaliana.PLoS One. 2012;7(10):e48443. doi: 10.1371/journal.pone.0048443. Epub 2012 Oct 29. PLoS One. 2012. PMID: 23144763 Free PMC article.
-
Regulatory Dynamics of Plant Hormones and Transcription Factors under Salt Stress.Biology (Basel). 2024 Aug 29;13(9):673. doi: 10.3390/biology13090673. Biology (Basel). 2024. PMID: 39336100 Free PMC article. Review.
-
Genome-Wide Identification, Phylogenetic, and Expression Analysis of Jasmonate ZIM-Domain Gene Family in Medicago Sativa L.Int J Mol Sci. 2024 Oct 1;25(19):10589. doi: 10.3390/ijms251910589. Int J Mol Sci. 2024. PMID: 39408917 Free PMC article.
References
-
- Kazan K, Manners JM. The interplay between light and jasmonate signalling during defence and development. J Exp Bot. 2011;62:4087–4100. - PubMed
-
- Kazan K, Manners JM. JAZ repressors and the orchestration of phytohormone crosstalk. Trends Plant Sci. 2012;17:22–31. - PubMed
-
- Browse J. Jasmonate passes muster: A receptor and targets for the defense hormone. Annu Rev Plant Biol. 2009;60:183–205. - PubMed
-
- Fonseca S, et al. (+)-7-iso-Jasmonoyl-L-isoleucine is the endogenous bioactive jasmonate. Nat Chem Biol. 2009;5:344–350. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

