ATM-dependent phosphorylation of SNEVhPrp19/hPso4 is involved in extending cellular life span and suppression of apoptosis
- PMID: 22529335
- PMCID: PMC3371764
- DOI: 10.18632/aging.100452
ATM-dependent phosphorylation of SNEVhPrp19/hPso4 is involved in extending cellular life span and suppression of apoptosis
Abstract
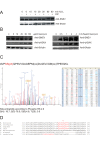
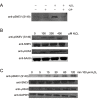
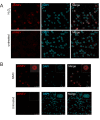
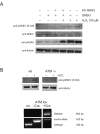
Defective DNA repair is widely acknowledged to negatively impact on healthy aging, since mutations in DNA repair factors lead to accelerated and premature aging. However, the opposite, namely if improved DNA repair will also increase the life or health span is less clear, and only few studies have tested if overexpression of DNA repair factors modulates life and health span in cells or organisms. Recently, we identified and characterized SNEVhPrp19/hPso4, a protein that plays a role in DNA repair and pre-mRNA splicing, and observed a doubling of the replicative life span upon ectopic overexpression, accompanied by lower basal DNA damage and apoptosis levels as well as an increased resistance to oxidative stress. Here we find that SNEVhPrp19/hPso4 is phosphorylated at S149 in an ataxia telangiectasia mutated protein (ATM)-dependent manner in response to oxidative stress and DNA double strand break inducing agents. By overexpressing wild-type SNEVhPrp19/hPso4 and a phosphorylation-deficient point-mutant, we found that S149 phosphorylation is necessary for mediating the resistance to apoptosis upon oxidative stress and is partially necessary for elongating the cellular life span. Therefore, ATM dependent phosphorylation of SNEVhPrp19/hPso4 upon DNA damage or oxidative stress might represent a novel axis capable of modulating cellular life span.
Conflict of interest statement
JG and RGV are co-founders of Evercyte GmbH.
Figures

Similar articles
-
Statins use a novel Nijmegen breakage syndrome-1-dependent pathway to accelerate DNA repair in vascular smooth muscle cells.Circ Res. 2008 Sep 26;103(7):717-25. doi: 10.1161/CIRCRESAHA.108.182899. Epub 2008 Aug 21. Circ Res. 2008. PMID: 18723444
-
Optimal function of the DNA repair enzyme TDP1 requires its phosphorylation by ATM and/or DNA-PK.EMBO J. 2009 Dec 2;28(23):3667-80. doi: 10.1038/emboj.2009.302. Epub 2009 Oct 22. EMBO J. 2009. PMID: 19851285 Free PMC article.
-
Nuclear ataxia-telangiectasia mutated (ATM) mediates the cellular response to DNA double strand breaks in human neuron-like cells.J Biol Chem. 2006 Jun 23;281(25):17482-17491. doi: 10.1074/jbc.M601895200. Epub 2006 Apr 20. J Biol Chem. 2006. PMID: 16627474
-
Activation and regulation of ATM kinase activity in response to DNA double-strand breaks.Oncogene. 2007 Dec 10;26(56):7741-8. doi: 10.1038/sj.onc.1210872. Oncogene. 2007. PMID: 18066086 Review.
-
The ATM-dependent DNA damage signaling pathway.Cold Spring Harb Symp Quant Biol. 2005;70:99-109. doi: 10.1101/sqb.2005.70.002. Cold Spring Harb Symp Quant Biol. 2005. PMID: 16869743 Review.
Cited by
-
The PRP19 Ubiquitin Ligase, Standing at the Cross-Roads of mRNA Processing and Genome Stability.Cancers (Basel). 2022 Feb 10;14(4):878. doi: 10.3390/cancers14040878. Cancers (Basel). 2022. PMID: 35205626 Free PMC article. Review.
-
Methylation of ribosomal RNA by NSUN5 is a conserved mechanism modulating organismal lifespan.Nat Commun. 2015 Jan 30;6:6158. doi: 10.1038/ncomms7158. Nat Commun. 2015. PMID: 25635753 Free PMC article.
-
Pre-mRNA processing factors meet the DNA damage response.Front Genet. 2013 Jun 6;4:102. doi: 10.3389/fgene.2013.00102. eCollection 2013. Front Genet. 2013. PMID: 23761808 Free PMC article.
-
Microsatellite Instability and Aberrant Pre-mRNA Splicing: How Intimate Is It?Genes (Basel). 2023 Jan 25;14(2):311. doi: 10.3390/genes14020311. Genes (Basel). 2023. PMID: 36833239 Free PMC article.
-
Ubiquitous overexpression of the DNA repair factor dPrp19 reduces DNA damage and extends Drosophila life span.NPJ Aging Mech Dis. 2017 Mar 15;3:5. doi: 10.1038/s41514-017-0005-z. eCollection 2017. NPJ Aging Mech Dis. 2017. PMID: 28649423 Free PMC article.
References
-
- Burtner C, Kennedy B. Progeria syndromes and ageing: what is the connection? Nat Rev Mol Cell Biol. 2010;11:567–578. - PubMed
-
- Schumacher B, Hoeijmakers J, Garinis G. Sealing the gap between nuclear DNA damage and longevity. Mol Cell Endocrinol. 2009;299:112–117. - PubMed
-
- Martin GM. Genetic modulation of senescent phenotypes in Homo sapiens. Cell. 2005;120:523–532. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

