CCN1: a novel inflammation-regulated biphasic immune cell migration modulator
- PMID: 22527715
- PMCID: PMC11114836
- DOI: 10.1007/s00018-012-0981-x
CCN1: a novel inflammation-regulated biphasic immune cell migration modulator
Abstract
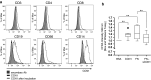
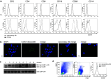
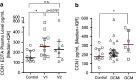
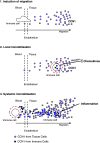
In this study, we performed a comprehensive analysis of the effect of CCN1 on the migration of human immune cells. The molecule CCN1, produced by fibroblasts and endothelial cells, is considered as an important matrix protein promoting tissue repair and immune cell adhesion by binding various integrins. We recently reported that CCN1 therapy is able to suppress acute inflammation in vivo. Here, we show that CCN1 binds to various immune cells including T cells, B cells, NK cells, and monocytes. The addition of CCN1 in vitro enhances both actin polymerization and transwell migration. Prolonged incubation with CCN1, however, results in the inhibition of migration of immune cells by a mechanism that involves downregulation of PI3Kγ, p38, and Akt activation. Furthermore, we observed that immune cells themselves produce constitutively CCN1 and secretion is induced by pro-inflammatory stimuli. In line with this finding, patients suffering from acute inflammation had enhanced serum levels of CCN1. These findings extend the classical concept of CCN1 as a locally produced cell matrix adhesion molecule and suggest that CCN1 plays an important role in regulating immune cell trafficking by attracting and locally immobilizing immune cells.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures



Similar articles
-
Matricellular protein CCN1/CYR61: a new player in inflammation and leukocyte trafficking.Semin Immunopathol. 2014 Mar;36(2):253-9. doi: 10.1007/s00281-014-0420-1. Epub 2014 Mar 18. Semin Immunopathol. 2014. PMID: 24638890 Review.
-
CCN1/Cyr61-PI3K/AKT signaling promotes retinal neovascularization in oxygen-induced retinopathy.Int J Mol Med. 2015 Dec;36(6):1507-18. doi: 10.3892/ijmm.2015.2371. Epub 2015 Oct 12. Int J Mol Med. 2015. PMID: 26459773 Free PMC article.
-
Matricellular signaling molecule CCN1 attenuates experimental autoimmune myocarditis by acting as a novel immune cell migration modulator.Circulation. 2010 Dec 21;122(25):2688-98. doi: 10.1161/CIRCULATIONAHA.110.945261. Epub 2010 Dec 6. Circulation. 2010. PMID: 21135363
-
CCN1 (CYR61) and CCN3 (NOV) signaling drives human trophoblast cells into senescence and stimulates migration properties.Cell Adh Migr. 2016 Mar 3;10(1-2):163-78. doi: 10.1080/19336918.2016.1139265. Epub 2016 Jan 8. Cell Adh Migr. 2016. PMID: 26744771 Free PMC article.
-
CCN1/CYR61: the very model of a modern matricellular protein.Cell Mol Life Sci. 2011 Oct;68(19):3149-63. doi: 10.1007/s00018-011-0778-3. Epub 2011 Jul 31. Cell Mol Life Sci. 2011. PMID: 21805345 Free PMC article. Review.
Cited by
-
Genome-wide mRNA expression profiling in vastus lateralis of COPD patients with low and normal fat free mass index and healthy controls.Respir Res. 2015 Jan 8;16(1):1. doi: 10.1186/s12931-014-0139-5. Respir Res. 2015. PMID: 25567521 Free PMC article.
-
Resonating with Cellular Pathways: Transcriptome Insights into Nonthermal Bioeffects of Middle Infrared Light Stimulation and Vibrational Strong Coupling on Cell Proliferation and Migration.Research (Wash D C). 2024 Apr 30;7:0353. doi: 10.34133/research.0353. eCollection 2024. Research (Wash D C). 2024. PMID: 38694203 Free PMC article.
-
A systematic review of multimodal clinical biomarkers in the management of thyroid eye disease.Rev Endocr Metab Disord. 2022 Jun;23(3):541-567. doi: 10.1007/s11154-021-09702-9. Epub 2022 Jan 23. Rev Endocr Metab Disord. 2022. PMID: 35066781
-
Epithelial and Endothelial Adhesion of Immune Cells Is Enhanced by Cardiotonic Steroid Signaling Through Na+/K+-ATPase-α-1.J Am Heart Assoc. 2020 Feb 4;9(3):e013933. doi: 10.1161/JAHA.119.013933. Epub 2020 Jan 30. J Am Heart Assoc. 2020. PMID: 32013704 Free PMC article.
-
CCN1 promotes IL-1β production in keratinocytes by activating p38 MAPK signaling in psoriasis.Sci Rep. 2017 Mar 7;7:43310. doi: 10.1038/srep43310. Sci Rep. 2017. PMID: 28266627 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

