Impaired dendritic cell maturation and IL-10 production following H. pylori stimulation in gastric cancer patients
- PMID: 22526791
- PMCID: PMC3433674
- DOI: 10.1007/s00253-012-4034-z
Impaired dendritic cell maturation and IL-10 production following H. pylori stimulation in gastric cancer patients
Abstract
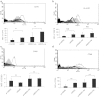
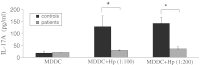
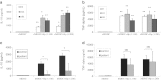
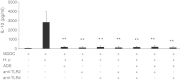
The current study was to investigate the interaction between Helicobacter pylori and human dendritic cells (DCs). Whether impaired DC function can influence the outcome of H. pylori infections. Human monocyte-derived DCs (MDDCs) from five gastric cancer patients and nine healthy controls were stimulated with H. pylori. Maturation markers of MDDC were examined by flow cytometry. IL-10 and TNF-α released by MDDCs and IL-17 produced by T cells were measured by ELISA. Regulatory signaling pathways of IL-10 were examined by ELISA, western blotting, and chromatin immunoprecipitation assay. The results showed that as compared with healthy individuals, the maturation marker CD40 in MDDCs, IL-17A expression from T cells, and IL-10 expression from MDDCs were significantly lower in gastric cancer patients. Blocking DC-SIGN, TLR2, and TLR4 could reverse H. pylori-associated IL-10 production. Activation of the p38 MAPK and NF-kB signaling pathways concomitant with decreased tri-methylated H3K9 and increased acetylated H3 accounted for the effect of H. pylori on IL-10 expression. Furthermore, upregulated IL-10 expression was significantly suppressed in H. pylori-pulsed MDDCs by histone acetyltransferase and methyltransferase inhibitors. Taken together, impaired DC function contributes to the less effective innate and adaptive immune responses against H. pylori seen in gastric cancer patients. H. pylori can regulate IL-10 production through Toll-like and DC-SIGN receptors, activates p-p38 MAPK signaling and the transcription factors NF-kB, and modulates histone modification.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Involvement of Toll-like receptors on Helicobacter pylori-induced immunity.PLoS One. 2014 Aug 25;9(8):e104804. doi: 10.1371/journal.pone.0104804. eCollection 2014. PLoS One. 2014. PMID: 25153703 Free PMC article.
-
Helicobacter pylori inhibits dendritic cell maturation via interleukin-10-mediated activation of the signal transducer and activator of transcription 3 pathway.J Innate Immun. 2015;7(2):199-211. doi: 10.1159/000368232. Epub 2014 Nov 20. J Innate Immun. 2015. PMID: 25412627 Free PMC article.
-
In vitro suppression of dendritic cells by Helicobacter pylori OipA.Helicobacter. 2014 Apr;19(2):136-43. doi: 10.1111/hel.12107. Epub 2014 Feb 5. Helicobacter. 2014. PMID: 24495278
-
Dendritic cell function in the host response to Helicobacter pylori infection of the gastric mucosa.Pathog Dis. 2013 Feb;67(1):46-53. doi: 10.1111/2049-632X.12014. Epub 2013 Jan 22. Pathog Dis. 2013. PMID: 23620119 Review.
-
Helicobacter pylori targets dendritic cells to induce immune tolerance, promote persistence and confer protection against allergic asthma.Gut Microbes. 2012 Nov-Dec;3(6):566-71. doi: 10.4161/gmic.21750. Epub 2012 Aug 16. Gut Microbes. 2012. PMID: 22895083 Free PMC article. Review.
Cited by
-
Involvement of Toll-like receptors on Helicobacter pylori-induced immunity.PLoS One. 2014 Aug 25;9(8):e104804. doi: 10.1371/journal.pone.0104804. eCollection 2014. PLoS One. 2014. PMID: 25153703 Free PMC article.
-
The current role of dendritic cells in the progression and treatment of colorectal cancer.Cancer Biol Med. 2024 Aug 22;21(9):769-83. doi: 10.20892/j.issn.2095-3941.2024.0188. Cancer Biol Med. 2024. PMID: 39177125 Free PMC article. Review.
-
Local Immune Response in Helicobacter pylori Infection.Medicine (Baltimore). 2016 May;95(20):e3713. doi: 10.1097/MD.0000000000003713. Medicine (Baltimore). 2016. PMID: 27196487 Free PMC article.
-
Function and subsets of dendritic cells and natural killer cells were decreased in gastric cancer.Int J Clin Exp Pathol. 2014 Oct 15;7(11):8304-11. eCollection 2014. Int J Clin Exp Pathol. 2014. PMID: 25550889 Free PMC article.
-
Lactobacillus curvatus WiKim38 isolated from kimchi induces IL-10 production in dendritic cells and alleviates DSS-induced colitis in mice.J Microbiol. 2016 Jul;54(7):503-9. doi: 10.1007/s12275-016-6160-2. Epub 2016 Jun 28. J Microbiol. 2016. PMID: 27350616
References
-
- Bergman MP, Engering A, Smits HH, van Vliet SJ, van Bodegraven AA, Wirth HP, Kapsenberg ML, Vandenbroucke-Grauls CM, van Kooyk Y, Appelmelk BJ. Helicobacter pylori modulates the T helper cell 1/T helper cell 2 balance through phase-variable interaction between lipopolysaccharide and DC-SIGN. J Exp Med. 2004;200:979–990. doi: 10.1084/jem.20041061. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

