Regulation of nuclear TDP-43 by NR2A-containing NMDA receptors and PTEN
- PMID: 22526419
- PMCID: PMC3336381
- DOI: 10.1242/jcs.095729
Regulation of nuclear TDP-43 by NR2A-containing NMDA receptors and PTEN
Abstract
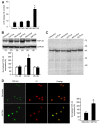
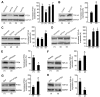
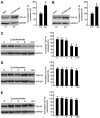
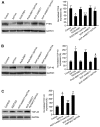
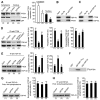
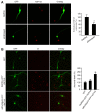
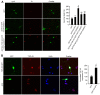
The dysfunction of TAR DNA-binding protein-43 (TDP-43) is implicated in neurodegenerative diseases. However, the function of TDP-43 is not fully elucidated. Here we show that the protein level of endogenous TDP-43 in the nucleus is increased in mouse cortical neurons in the early stages, but return to basal level in the later stages after glutamate accumulation-induced injury. The elevation of TDP-43 results from a downregulation of phosphatase and tensin homolog (PTEN). We further demonstrate that activation of NR2A-containing NMDA receptors (NR2ARs) leads to PTEN downregulation and subsequent reduction of PTEN import from the cytoplasm to the nucleus after glutamate accumulation. The decrease of PTEN in the nucleus contributes to its reduced association with TDP-43, and thereby mediates the elevation of nuclear TDP-43. We provide evidence that the elevation of nuclear TDP-43, mediated by NR2AR activation and PTEN downregulation, confers protection against cortical neuronal death in the late stages after glutamate accumulation. Thus, this study reveals a NR2AR-PTEN-TDP-43 signaling pathway by which nuclear TDP-43 promotes neuronal survival. These results suggest that upregulation of nuclear TDP-43 represents a self-protection mechanism to delay neurodegeneration in the early stages after glutamate accumulation and that prolonging the upregulation process of nuclear TDP-43 might have therapeutic significance.
Figures
Similar articles
-
Caspase-4 mediates cytoplasmic accumulation of TDP-43 in the primate brains.Acta Neuropathol. 2019 Jun;137(6):919-937. doi: 10.1007/s00401-019-01979-0. Epub 2019 Feb 27. Acta Neuropathol. 2019. PMID: 30810811 Free PMC article.
-
Coaggregation of RNA-binding proteins in a model of TDP-43 proteinopathy with selective RGG motif methylation and a role for RRM1 ubiquitination.PLoS One. 2012;7(6):e38658. doi: 10.1371/journal.pone.0038658. Epub 2012 Jun 21. PLoS One. 2012. PMID: 22761693 Free PMC article.
-
Identification of neuronal RNA targets of TDP-43-containing ribonucleoprotein complexes.J Biol Chem. 2011 Jan 14;286(2):1204-15. doi: 10.1074/jbc.M110.190884. Epub 2010 Nov 4. J Biol Chem. 2011. PMID: 21051541 Free PMC article.
-
TDP-43 in aging and Alzheimer's disease - a review.Int J Clin Exp Pathol. 2011 Jan 30;4(2):147-55. Int J Clin Exp Pathol. 2011. PMID: 21326809 Free PMC article. Review.
-
TDP-43 proteinopathy and mitochondrial abnormalities in neurodegeneration.Mol Cell Neurosci. 2019 Oct;100:103396. doi: 10.1016/j.mcn.2019.103396. Epub 2019 Aug 21. Mol Cell Neurosci. 2019. PMID: 31445085 Free PMC article. Review.
Cited by
-
A synthetic BBB-permeable tripeptide GCF confers neuroprotection by increasing glycine in the ischemic brain.Front Pharmacol. 2022 Aug 15;13:950376. doi: 10.3389/fphar.2022.950376. eCollection 2022. Front Pharmacol. 2022. PMID: 36046828 Free PMC article.
-
NMDA Receptor C-Terminal Domain Signalling in Development, Maturity, and Disease.Int J Mol Sci. 2022 Sep 27;23(19):11392. doi: 10.3390/ijms231911392. Int J Mol Sci. 2022. PMID: 36232696 Free PMC article. Review.
-
Glycine confers neuroprotection through microRNA-301a/PTEN signaling.Mol Brain. 2016 May 26;9(1):59. doi: 10.1186/s13041-016-0241-3. Mol Brain. 2016. PMID: 27230112 Free PMC article.
-
A network of RNA and protein interactions in Fronto Temporal Dementia.Front Mol Neurosci. 2015 Mar 19;8:9. doi: 10.3389/fnmol.2015.00009. eCollection 2015. Front Mol Neurosci. 2015. PMID: 25852467 Free PMC article. Review.
-
The Functional and Molecular Properties, Physiological Functions, and Pathophysiological Roles of GluN2A in the Central Nervous System.Mol Neurobiol. 2017 Mar;54(2):1008-1021. doi: 10.1007/s12035-016-9715-7. Epub 2016 Jan 21. Mol Neurobiol. 2017. PMID: 26797520 Review.
References
-
- Alilain W. J., Goshgarian H. G. (2008). Glutamate receptor plasticity and activity-regulated cytoskeletal associated protein regulation in the phrenic motor nucleus may mediate spontaneous recovery of the hemidiaphragm following chronic cervical spinal cord injury. Exp. Neurol. 212, 348-357 - PMC - PubMed
-
- Annis C. M., Vaughn J. E. (1998). Differential vulnerability of autonomic and somatic motor neurons to N-methyl-D-aspartate-induced excitotoxicity. Neuroscience 83, 239-249 - PubMed
-
- Arai T., Hasegawa M., Akiyama H., Ikeda K., Nonaka T., Mori H., Mann D., Tsuchiya K., Yoshida M., Hashizume Y., et al. (2006). TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem. Biophys. Res. Commun. 351, 602-611 - PubMed
-
- Arai T., Mackenzie I. R., Hasegawa M., Nonoka T., Niizato K., Tsuchiya K., Iritani S., Onaya M., Akiyama H. (2009). Phosphorylated TDP-43 in Alzheimer's disease and dementia with Lewy bodies. Acta Neuropathol. 117, 125-136 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

