Calpain and STriatal-Enriched protein tyrosine phosphatase (STEP) activation contribute to extrasynaptic NMDA receptor localization in a Huntington's disease mouse model
- PMID: 22523092
- PMCID: PMC3412376
- DOI: 10.1093/hmg/dds154
Calpain and STriatal-Enriched protein tyrosine phosphatase (STEP) activation contribute to extrasynaptic NMDA receptor localization in a Huntington's disease mouse model
Abstract
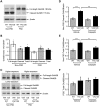
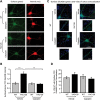
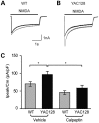
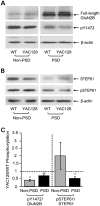
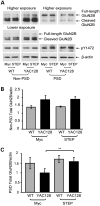
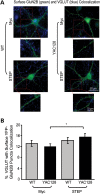
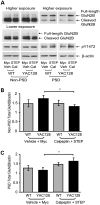
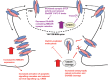
In Huntington's disease (HD), the mutant huntingtin (mhtt) protein is associated with striatal dysfunction and degeneration. Excitotoxicity and early synaptic defects are attributed, in part, to altered NMDA receptor (NMDAR) trafficking and function. Deleterious extrasynaptic NMDAR localization and signalling are increased early in yeast artificial chromosome mice expressing full-length mhtt with 128 polyglutamine repeats (YAC128 mice). NMDAR trafficking at the plasma membrane is regulated by dephosphorylation of the NMDAR subunit GluN2B tyrosine 1472 (Y1472) residue by STriatal-Enriched protein tyrosine Phosphatase (STEP). NMDAR function is also regulated by calpain cleavage of the GluN2B C-terminus. Activation of both STEP and calpain is calcium-dependent, and disruption of calcium homeostasis occurs early in the HD striatum. Here, we show increased calpain cleavage of GluN2B at both synaptic and extrasynaptic sites, and elevated extrasynaptic total GluN2B expression in the YAC128 striatum. Calpain inhibition significantly reduced extrasynaptic GluN2B expression in the YAC128 but not wild-type striatum. Furthermore, calpain inhibition reduced whole-cell NMDAR current and the surface/internal GluN2B ratio in co-cultured striatal neurons, without affecting synaptic GluN2B localization. Synaptic STEP activity was also significantly higher in the YAC128 striatum, correlating with decreased GluN2B Y1472 phosphorylation. A substrate-trapping STEP protein (TAT-STEP C-S) significantly increased VGLUT1-GluN2B colocalization, as well as increasing synaptic GluN2B expression and Y1472 phosphorylation. Moreover, combined calpain inhibition and STEP inactivation reduced extrasynaptic, while increasing synaptic GluN2B expression in the YAC128 striatum. These results indicate that increased STEP and calpain activation contribute to altered NMDAR localization in an HD mouse model, suggesting new therapeutic targets for HD.
Figures
Similar articles
-
Alterations in STriatal-Enriched protein tyrosine Phosphatase expression, activation, and downstream signaling in early and late stages of the YAC128 Huntington's disease mouse model.J Neurochem. 2014 Jul;130(1):145-59. doi: 10.1111/jnc.12700. Epub 2014 Apr 2. J Neurochem. 2014. PMID: 24588402 Free PMC article.
-
P38 MAPK is involved in enhanced NMDA receptor-dependent excitotoxicity in YAC transgenic mouse model of Huntington disease.Neurobiol Dis. 2012 Mar;45(3):999-1009. doi: 10.1016/j.nbd.2011.12.019. Epub 2011 Dec 14. Neurobiol Dis. 2012. PMID: 22198502
-
Mitigation of augmented extrasynaptic NMDAR signaling and apoptosis in cortico-striatal co-cultures from Huntington's disease mice.Neurobiol Dis. 2012 Oct;48(1):40-51. doi: 10.1016/j.nbd.2012.05.013. Epub 2012 Jun 2. Neurobiol Dis. 2012. PMID: 22668780
-
Pathophysiology of Huntington's disease: time-dependent alterations in synaptic and receptor function.Neuroscience. 2011 Dec 15;198:252-73. doi: 10.1016/j.neuroscience.2011.08.052. Epub 2011 Aug 27. Neuroscience. 2011. PMID: 21907762 Free PMC article. Review.
-
Role of nonsynaptic GluN2B-containing NMDA receptors in excitotoxicity: evidence that fluoxetine selectively inhibits these receptors and may have neuroprotective effects.Brain Res Bull. 2013 Apr;93:32-8. doi: 10.1016/j.brainresbull.2012.10.005. Epub 2012 Oct 23. Brain Res Bull. 2013. PMID: 23089362 Review.
Cited by
-
Upregulation of calpain activity precedes tau phosphorylation and loss of synaptic proteins in Alzheimer's disease brain.Acta Neuropathol Commun. 2016 Mar 31;4:34. doi: 10.1186/s40478-016-0299-2. Acta Neuropathol Commun. 2016. PMID: 27036949 Free PMC article.
-
Pyk2 modulates hippocampal excitatory synapses and contributes to cognitive deficits in a Huntington's disease model.Nat Commun. 2017 May 30;8:15592. doi: 10.1038/ncomms15592. Nat Commun. 2017. PMID: 28555636 Free PMC article.
-
From pathways to targets: understanding the mechanisms behind polyglutamine disease.Biomed Res Int. 2014;2014:701758. doi: 10.1155/2014/701758. Epub 2014 Sep 21. Biomed Res Int. 2014. PMID: 25309920 Free PMC article. Review.
-
DAPK1 Promotes Extrasynaptic GluN2B Phosphorylation and Striatal Spine Instability in the YAC128 Mouse Model of Huntington Disease.Front Cell Neurosci. 2020 Nov 5;14:590569. doi: 10.3389/fncel.2020.590569. eCollection 2020. Front Cell Neurosci. 2020. PMID: 33250715 Free PMC article.
-
Role of NMDA Receptor-Mediated Glutamatergic Signaling in Chronic and Acute Neuropathologies.Neural Plast. 2016;2016:2701526. doi: 10.1155/2016/2701526. Epub 2016 Aug 18. Neural Plast. 2016. PMID: 27630777 Free PMC article. Review.
References
-
- Bliss T.V., Collingridge G.L. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi:10.1038/361031a0. - DOI - PubMed
-
- Arundine M., Tymianski M. Molecular mechanisms of calcium-dependent neurodegeneration in excitotoxicity. Cell Calcium. 2003;34:325–337. doi:10.1016/S0143-4160(03)00141-6. - DOI - PubMed
-
- Gladding C.M., Raymond L.A. Mechanisms underlying NMDA receptor synaptic/extrasynaptic distribution and function. Mol. Cell Neurosci. 2011;48:308–320. - PubMed
-
- Lau C.G., Zukin R.S. NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat. Rev. Neurosci. 2007;8:413–426. - PubMed
-
- Graveland G.A., Williams R.S., DiFiglia M. Evidence for degenerative and regenerative changes in neostriatal spiny neurons in Huntington's disease. Science. 1985;227:770–773. doi:10.1126/science.3155875. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

