Continuous estrone treatment impairs spatial memory and does not impact number of basal forebrain cholinergic neurons in the surgically menopausal middle-aged rat
- PMID: 22522079
- PMCID: PMC3397199
- DOI: 10.1016/j.yhbeh.2012.04.004
Continuous estrone treatment impairs spatial memory and does not impact number of basal forebrain cholinergic neurons in the surgically menopausal middle-aged rat
Abstract
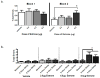
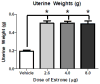
CEE (conjugated equine estrogens) is the most widely prescribed estrogen-only menopausal hormone therapy in the United States, and is comprised of over 50% estrone (E1) sulfate. Following CEE administration, E1 is the principal circulating estrogen. However, the cognitive and neurobiological effects of E1 in a middle-aged rodent model have not yet been evaluated. We assessed cognitive effects of continuous E1 treatment in middle-aged surgically menopausal rats using a maze battery. We also quantified number of choline acetyltransferase-immunoreactive (ChAT-IR) neurons in distinct basal forebrain regions known in earlier studies in to be impacted by the most potent naturally-circulating estrogen in rodents and women, 17β-estradiol (17β-E2), as well as CEE. On the spatial working memory delayed-match-to-sample water maze, the highest E1 dose impaired memory performance during acquisition and after delay challenge. E1 did not impact ChAT-IR neuron number in the medial septum (MS) or horizontal/vertical diagonal bands. In a comparison study, 17β-E2 increased MS ChAT-IR neuron number. Findings indicate that E1 negatively impacts spatial working memory and memory retention, and does not increase ChAT-IR neuron number in basal forebrain, as does 17β-E2. Thus, data from prior studies suggest that 17β-E2 and CEE can enhance cognition and increase number of ChAT-IR basal forebrain neurons, while here we show that E1 does not induce these effects. Findings from preclinical basic science studies can inform the design of specific combinations of estrogens that could be beneficial to the brain and cognition. Accumulating data suggest that E1 is not likely to be among these key beneficial estrogens.
Published by Elsevier Inc.
Figures

Similar articles
-
Premarin improves memory, prevents scopolamine-induced amnesia and increases number of basal forebrain choline acetyltransferase positive cells in middle-aged surgically menopausal rats.Horm Behav. 2009 Mar;55(3):454-64. doi: 10.1016/j.yhbeh.2008.11.008. Epub 2008 Dec 3. Horm Behav. 2009. PMID: 19101559 Free PMC article.
-
Understanding the cognitive impact of the contraceptive estrogen Ethinyl Estradiol: tonic and cyclic administration impairs memory, and performance correlates with basal forebrain cholinergic system integrity.Psychoneuroendocrinology. 2015 Apr;54:1-13. doi: 10.1016/j.psyneuen.2015.01.002. Epub 2015 Jan 12. Psychoneuroendocrinology. 2015. PMID: 25679306 Free PMC article.
-
A component of Premarin(®) enhances multiple cognitive functions and influences nicotinic receptor expression.Horm Behav. 2010 Nov;58(5):917-28. doi: 10.1016/j.yhbeh.2010.09.002. Epub 2010 Sep 19. Horm Behav. 2010. PMID: 20849857 Free PMC article.
-
Reprint of: From the 90׳s to now: A brief historical perspective on more than two decades of estrogen neuroprotection.Brain Res. 2016 Aug 15;1645:79-82. doi: 10.1016/j.brainres.2016.06.016. Epub 2016 Jun 16. Brain Res. 2016. PMID: 27317847 Free PMC article. Review.
-
Estrogens and menopause: pharmacology of conjugated equine estrogens and their potential role in the prevention of neurodegenerative diseases such as Alzheimer's.J Steroid Biochem Mol Biol. 2003 Jun;85(2-5):473-82. doi: 10.1016/s0960-0760(03)00220-6. J Steroid Biochem Mol Biol. 2003. PMID: 12943738 Review.
Cited by
-
Oestrogen treatment modulates the impact of cognitive experience and task complexity on memory in middle-aged surgically menopausal rats.J Neuroendocrinol. 2021 Jun 18;33(9):e13002. doi: 10.1111/jne.13002. Online ahead of print. J Neuroendocrinol. 2021. PMID: 34378820 Free PMC article.
-
Estrogens as neuroprotectants: Estrogenic actions in the context of cognitive aging and brain injury.Prog Neurobiol. 2017 Oct;157:188-211. doi: 10.1016/j.pneurobio.2015.12.008. Epub 2016 Feb 15. Prog Neurobiol. 2017. PMID: 26891883 Free PMC article. Review.
-
The endocrine-brain-aging triad where many paths meet: female reproductive hormone changes at midlife and their influence on circuits important for learning and memory.Exp Gerontol. 2017 Aug;94:14-23. doi: 10.1016/j.exger.2016.12.011. Epub 2016 Dec 13. Exp Gerontol. 2017. PMID: 27979770 Free PMC article.
-
Is Hormone Replacement Therapy a Risk Factor or a Therapeutic Option for Alzheimer's Disease?Int J Mol Sci. 2023 Feb 6;24(4):3205. doi: 10.3390/ijms24043205. Int J Mol Sci. 2023. PMID: 36834617 Free PMC article. Review.
-
Gynecological surgery in adulthood imparts cognitive and brain changes in rats: A focus on hysterectomy at short-, moderate-, and long-term intervals after surgery.Horm Behav. 2023 Sep;155:105411. doi: 10.1016/j.yhbeh.2023.105411. Epub 2023 Aug 31. Horm Behav. 2023. PMID: 37659358 Free PMC article.
References
-
- Acosta JI, Mayer L, Talboom JS, Zay C, Scheldrup M, Castillo J, Demers LM, Enders CK, Bimonte-Nelson HA. Premarin improves memory, prevents scopolamine-induced amnesia and increases number of basal forebrain choline acetyltransferase positive cells in middle-aged surgically menopausal rats. Horm Behav. 2009b;55:454–464. - PMC - PubMed
-
- Beyer C, Morali G, Larsson K, Södersten P. Steroid regulation of sexual behavior. Journal of Steroid Biochemistry. 1976;7:1171–1176. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

