The solute carrier 6 family of transporters
- PMID: 22519513
- PMCID: PMC3481037
- DOI: 10.1111/j.1476-5381.2012.01975.x
The solute carrier 6 family of transporters
Abstract
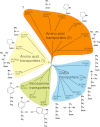
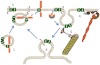
The solute carrier 6 (SLC6) family of the human genome comprises transporters for neurotransmitters, amino acids, osmolytes and energy metabolites. Members of this family play critical roles in neurotransmission, cellular and whole body homeostasis. Malfunction or altered expression of these transporters is associated with a variety of diseases. Pharmacological inhibition of the neurotransmitter transporters in this family is an important strategy in the management of neurological and psychiatric disorders. This review provides an overview of the biochemical and pharmacological properties of the SLC6 family transporters.
© 2012 The Authors. British Journal of Pharmacology © 2012 The British Pharmacological Society.
Figures

Similar articles
-
Synaptic uptake and beyond: the sodium- and chloride-dependent neurotransmitter transporter family SLC6.Pflugers Arch. 2004 Feb;447(5):519-31. doi: 10.1007/s00424-003-1064-5. Epub 2003 Apr 29. Pflugers Arch. 2004. PMID: 12719981 Review.
-
The SLC6 orphans are forming a family of amino acid transporters.Neurochem Int. 2006 May-Jun;48(6-7):559-67. doi: 10.1016/j.neuint.2005.11.021. Epub 2006 Mar 15. Neurochem Int. 2006. PMID: 16540203 Review.
-
SLC6 transporters: structure, function, regulation, disease association and therapeutics.Mol Aspects Med. 2013 Apr-Jun;34(2-3):197-219. doi: 10.1016/j.mam.2012.07.002. Mol Aspects Med. 2013. PMID: 23506866 Free PMC article. Review.
-
Getting the message across: a recent transporter structure shows the way.Neuron. 2006 Mar 16;49(6):791-6. doi: 10.1016/j.neuron.2006.03.002. Neuron. 2006. PMID: 16543127 Review.
-
SLC6 neurotransmitter transporters: structure, function, and regulation.Pharmacol Rev. 2011 Sep;63(3):585-640. doi: 10.1124/pr.108.000869. Epub 2011 Jul 13. Pharmacol Rev. 2011. PMID: 21752877 Review.
Cited by
-
SLC6A14 facilitates epithelial cell ferroptosis via the C/EBPβ-PAK6 axis in ulcerative colitis.Cell Mol Life Sci. 2022 Oct 22;79(11):563. doi: 10.1007/s00018-022-04594-7. Cell Mol Life Sci. 2022. PMID: 36272033 Free PMC article.
-
Spontaneous inward opening of the dopamine transporter is triggered by PIP2-regulated dynamics of the N-terminus.ACS Chem Neurosci. 2015 Nov 18;6(11):1825-37. doi: 10.1021/acschemneuro.5b00179. Epub 2015 Aug 17. ACS Chem Neurosci. 2015. PMID: 26255829 Free PMC article.
-
Illuminating the monoamine transporters: Fluorescently labelled ligands to study dopamine, serotonin and norepinephrine transporters.Basic Clin Pharmacol Toxicol. 2023 Nov;133(5):473-484. doi: 10.1111/bcpt.13827. Epub 2023 Jan 7. Basic Clin Pharmacol Toxicol. 2023. PMID: 36527444 Free PMC article. Review.
-
Thermostabilization and purification of the human dopamine transporter (hDAT) in an inhibitor and allosteric ligand bound conformation.PLoS One. 2018 Jul 2;13(7):e0200085. doi: 10.1371/journal.pone.0200085. eCollection 2018. PLoS One. 2018. PMID: 29965988 Free PMC article.
-
Identification and characterization of the Fasciola hepatica sodium- and chloride-dependent taurine transporter.PLoS Negl Trop Dis. 2018 Apr 27;12(4):e0006428. doi: 10.1371/journal.pntd.0006428. eCollection 2018 Apr. PLoS Negl Trop Dis. 2018. PMID: 29702654 Free PMC article.
References
-
- Agoston GE, Wu JH, Izenwasser S, George C, Katz J, Kline RH, et al. Novel N-substituted 3 alpha-[bis(4′-fluorophenyl)methoxy]tropane analogues: selective ligands for the dopamine transporter. J Med Chem. 1997;40:4329–4339. - PubMed
-
- Andersen J, Kristensen AS, Bang-Andersen B, Stromgaard K. Recent advances in the understanding of the interaction of antidepressant drugs with serotonin and norepinephrine transporters. Chem Commun (Camb) 2009:3677–3692. 2009. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

