Calcium-sensing receptor inhibits secretagogue-induced electrolyte secretion by intestine via the enteric nervous system
- PMID: 22517767
- PMCID: PMC3404579
- DOI: 10.1152/ajpgi.00425.2011
Calcium-sensing receptor inhibits secretagogue-induced electrolyte secretion by intestine via the enteric nervous system
Abstract
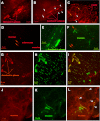
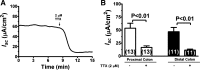
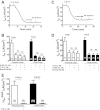
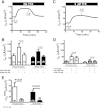
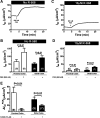
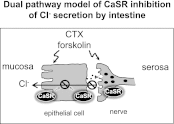
Bacterial toxins such as cholera toxin induce diarrhea by both direct epithelial cell generation of cyclic nucleotides as well as stimulation of the enteric nervous system (ENS). Agonists of the extracellular calcium-sensing receptor (CaSR) can reduce toxin-stimulated fluid secretion in ENS-absent colonic epithelial crypts by increasing phosphodiesterase-dependent cyclic-nucleotide degradation. Here we show that the CaSR is also highly expressed in tetrodotoxin (TTX)-sensitive neurons comprising the ENS, suggesting that CaSR agonists might also function through neuronal pathways. To test this hypothesis, rat colon segments containing intact ENS were isolated and mounted on Ussing chambers. Basal and cyclic nucleotide-stimulated electrolyte secretions were monitored by measuring changes in short-circuit current (I(sc)). CaSR was activated by R-568 and its effects were compared in the presence and absence of TTX. Consistent with active regulation of anion secretion by the ENS, a significant proportion of I(sc) in the proximal and distal colon was inhibited by serosal TTX, both at basal and under cyclic AMP-stimulated conditions. In the absence of TTX, activation of CaSR with R-568 significantly reduced basal I(sc) and cyclic AMP-stimulated I(sc); it also completely reversed the cAMP-stimulated secretory responses if the drug was applied after the forskolin stimulation. Such inhibitory effects of R-568 were either absent or significantly reduced when serosal TTX was present, suggesting that this agonist exerts its antisecretory effect on the intestine by inhibiting ENS. The present results suggest a new model for regulating intestinal fluid transport in which neuronal and nonneuronal secretagogue actions are modulated by the inhibitory effects of CaSR on the ENS. The ability of a CaSR agonist to reduce secretagogue-stimulated Cl(-) secretion might provide a new therapeutic approach for secretory and other ENS-mediated diarrheal conditions.
Figures



Similar articles
-
Calcimimetic R568 inhibits tetrodotoxin-sensitive colonic electrolyte secretion and reduces c-fos expression in myenteric neurons.Life Sci. 2018 Feb 1;194:49-58. doi: 10.1016/j.lfs.2017.12.019. Epub 2017 Dec 13. Life Sci. 2018. PMID: 29247746 Free PMC article.
-
Calcimimetic acts on enteric neuronal CaSR to reverse cholera toxin-induced intestinal electrolyte secretion.Sci Rep. 2018 May 18;8(1):7851. doi: 10.1038/s41598-018-26171-4. Sci Rep. 2018. PMID: 29777154 Free PMC article.
-
Calcium-sensing receptor stimulates Cl(-)- and SCFA-dependent but inhibits cAMP-dependent HCO3(-) secretion in colon.Am J Physiol Gastrointest Liver Physiol. 2015 May 15;308(10):G874-83. doi: 10.1152/ajpgi.00341.2014. Epub 2015 Mar 19. Am J Physiol Gastrointest Liver Physiol. 2015. PMID: 25792563 Free PMC article.
-
The enteric nervous system and cholera toxin-induced secretion.Comp Biochem Physiol A Physiol. 1997 Oct;118(2):319-27. doi: 10.1016/s0300-9629(96)00312-x. Comp Biochem Physiol A Physiol. 1997. PMID: 9366063 Review.
-
Translating molecular physiology of intestinal transport into pharmacologic treatment of diarrhea: stimulation of Na+ absorption.Clin Gastroenterol Hepatol. 2014 Jan;12(1):27-31. doi: 10.1016/j.cgh.2013.10.020. Epub 2013 Oct 31. Clin Gastroenterol Hepatol. 2014. PMID: 24184676 Free PMC article. Review.
Cited by
-
Extracellular Calcium Dictates Onset, Severity, and Recovery of Diarrhea in a Child with Immune-Mediated Enteropathy.Front Pediatr. 2018 Jan 29;6:7. doi: 10.3389/fped.2018.00007. eCollection 2018. Front Pediatr. 2018. PMID: 29435439 Free PMC article.
-
Inability to reduce morbidity of diarrhea by ORS: can we design a better therapy?Pediatr Res. 2018 Mar;83(3):559-563. doi: 10.1038/pr.2017.295. Epub 2018 Jan 3. Pediatr Res. 2018. PMID: 29168980 Free PMC article. Review.
-
The Extracellular Calcium-Sensing Receptor in the Intestine: Evidence for Regulation of Colonic Absorption, Secretion, Motility, and Immunity.Front Physiol. 2016 Jun 21;7:245. doi: 10.3389/fphys.2016.00245. eCollection 2016. Front Physiol. 2016. PMID: 27458380 Free PMC article. Review.
-
Chloride channel-targeted therapy for secretory diarrheas.Curr Opin Pharmacol. 2013 Dec;13(6):888-94. doi: 10.1016/j.coph.2013.08.005. Epub 2013 Aug 27. Curr Opin Pharmacol. 2013. PMID: 23992767 Free PMC article. Review.
-
The calcium-sensing receptor: A novel target for treatment and prophylaxis of neratinib-induced diarrhea.Pharmacol Res Perspect. 2019 Sep 13;7(5):e00521. doi: 10.1002/prp2.521. eCollection 2019 Oct. Pharmacol Res Perspect. 2019. PMID: 31523434 Free PMC article.
References
-
- Alam Mu Kirton J, Wilkinson F, Towers E, Sinha S, Rouhi M, Vizard T, Sage A, Martin D, Ward D, Alexander M, Riccardi D, Canfield A. Calcification is associated with loss of functional calcium-sensing receptor in vascular smooth muscle cells. Cardiovasc Res 81: 260– 268, 2009 - PubMed
-
- Black R, Cousens S, Johnson H, Lawn J, Rudan I, Bassani D, Jha P, Campbell H, Walker C, Cibulskis R, Eisele T, Liu L, Mathers C; Child Health Epidemiology Reference Group of WHO and UNICEF Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet 375: 1969– 1987, 2010 - PubMed
-
- Bovee-Oudenhoven IMJ, Lettink-Wissink MLG, Van Doesburg W, Witteman BJM, Van Der Meer R. Diarrhea caused by enterotoxigenic Escherichia coli infection of humans is inhibited by dietary calcium. Gastroenterology 125: 469– 476, 2003 - PubMed
-
- Brown EM, Gamba G, Riccardi D, Lombardi M, Butters R, Kifor O, Sun A, Hediger MA, Lytton J, Hebert SC. Cloning and characterization of an extracellular Ca2+-sensing receptor from bovine parathyroid. Nature 366: 575– 580, 1993 - PubMed
-
- Brown EM, MacLeod RJ. Extracellular calcium sensing and extracellular calcium signaling. Physiol Rev 81: 239– 297, 2001 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

