Butyrate suppresses colonic inflammation through HDAC1-dependent Fas upregulation and Fas-mediated apoptosis of T cells
- PMID: 22517765
- PMCID: PMC3378095
- DOI: 10.1152/ajpgi.00543.2011
Butyrate suppresses colonic inflammation through HDAC1-dependent Fas upregulation and Fas-mediated apoptosis of T cells
Abstract
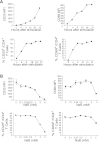
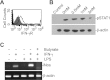
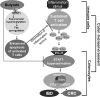
Butyrate, an intestinal microbiota metabolite of dietary fiber, has been shown to exhibit protective effects toward inflammatory diseases such as ulcerative colitis (UC) and inflammation-mediated colorectal cancer. Recent studies have shown that chronic IFN-γ signaling plays an essential role in inflammation-mediated colorectal cancer development in vivo, whereas genome-wide association studies have linked human UC risk loci to IFNG, the gene that encodes IFN-γ. However, the molecular mechanisms underlying the butyrate-IFN-γ-colonic inflammation axis are not well defined. Here we showed that colonic mucosa from patients with UC exhibit increased signal transducer and activator of transcription 1 (STAT1) activation, and this STAT1 hyperactivation is correlated with increased T cell infiltration. Butyrate treatment-induced apoptosis of wild-type T cells but not Fas-deficient (Fas(lpr)) or FasL-deficient (Fas(gld)) T cells, revealing a potential role of Fas-mediated apoptosis of T cells as a mechanism of butyrate function. Histone deacetylase 1 (HDAC1) was found to bind to the Fas promoter in T cells, and butyrate inhibits HDAC1 activity to induce Fas promoter hyperacetylation and Fas upregulation in T cells. Knocking down gpr109a or slc5a8, the genes that encode for receptor and transporter of butyrate, respectively, resulted in altered expression of genes related to multiple inflammatory signaling pathways, including inducible nitric oxide synthase (iNOS), in mouse colonic epithelial cells in vivo. Butyrate effectively inhibited IFN-γ-induced STAT1 activation, resulting in inhibition of iNOS upregulation in human colon epithelial and carcinoma cells in vitro. Our data thus suggest that butyrate delivers a double-hit: induction of T cell apoptosis to eliminate the source of inflammation and suppression of IFN-γ-mediated inflammation in colonic epithelial cells, to suppress colonic inflammation.
Figures





Similar articles
-
Essential role of the JAK/STAT1 signaling pathway in the expression of inducible nitric-oxide synthase in intestinal epithelial cells and its regulation by butyrate.J Biol Chem. 2007 Mar 30;282(13):9797-9804. doi: 10.1074/jbc.M609426200. Epub 2007 Jan 24. J Biol Chem. 2007. PMID: 17251186
-
Fas activates the JNK pathway in human colonic epithelial cells: lack of a direct role in apoptosis.Am J Physiol. 1999 Mar;276(3):G599-605. doi: 10.1152/ajpgi.1999.276.3.G599. Am J Physiol. 1999. PMID: 10070035
-
Inhibition of interferon gamma signaling by the short chain fatty acid butyrate.Mol Cancer Res. 2003 Sep;1(11):855-62. Mol Cancer Res. 2003. PMID: 14517348
-
Development, validation and implementation of an in vitro model for the study of metabolic and immune function in normal and inflamed human colonic epithelium.Dan Med J. 2015 Jan;62(1):B4973. Dan Med J. 2015. PMID: 25557335 Review.
-
Review article: the role of butyrate on colonic function.Aliment Pharmacol Ther. 2008 Jan 15;27(2):104-19. doi: 10.1111/j.1365-2036.2007.03562.x. Epub 2007 Oct 25. Aliment Pharmacol Ther. 2008. PMID: 17973645 Review.
Cited by
-
Investigating the Influence of Gut Microbiota-related Metabolites in Gastrointestinal Cancer.Curr Cancer Drug Targets. 2024;24(6):612-628. doi: 10.2174/0115680096274860231111210214. Curr Cancer Drug Targets. 2024. PMID: 38213140 Review.
-
Increased abundance of Firmicutes and depletion of Bacteroidota predicts poor outcome in chronic lymphocytic leukemia.Oncol Lett. 2024 Sep 17;28(5):552. doi: 10.3892/ol.2024.14685. eCollection 2024 Nov. Oncol Lett. 2024. PMID: 39328278 Free PMC article.
-
Combined Omics Analysis Further Unveils the Specific Role of Butyrate in Promoting Growth in Early-Weaning Animals.Int J Mol Sci. 2023 Jan 16;24(2):1787. doi: 10.3390/ijms24021787. Int J Mol Sci. 2023. PMID: 36675302 Free PMC article.
-
Impact of the Exposome on the Epigenome in Inflammatory Bowel Disease Patients and Animal Models.Int J Mol Sci. 2022 Jul 9;23(14):7611. doi: 10.3390/ijms23147611. Int J Mol Sci. 2022. PMID: 35886959 Free PMC article. Review.
-
Advances in treatment of ulcerative colitis with herbs: from bench to bedside.World J Gastroenterol. 2014 Oct 21;20(39):14099-104. doi: 10.3748/wjg.v20.i39.14099. World J Gastroenterol. 2014. PMID: 25339799 Free PMC article. Review.
References
-
- Anderson CA, Boucher G, Lees CW, Franke A, D'Amato M, Taylor KD, Lee JC, Goyette P, Imielinski M, Latiano A, Lagace C, Scott R, Amininejad L, Bumpstead S, Baidoo L, Baldassano RN, Barclay M, Bayless TM, Brand S, Buning C, Colombel JF, Denson LA, De Vos M, Dubinsky M, Edwards C, Ellinghaus D, Fehrmann RS, Floyd JA, Florin T, Franchimont D, Franke L, Georges M, Glas J, Glazer NL, Guthery SL, Haritunians T, Hayward NK, Hugot JP, Jobin G, Laukens D, Lawrance I, Lemann M, Levine A, Libioulle C, Louis E, McGovern DP, Milla M, Montgomery GW, Morley KI, Mowat C, Ng A, Newman W, Ophoff RA, Papi L, Palmieri O, Peyrin-Biroulet L, Panes J, Phillips A, Prescott NJ, Proctor DD, Roberts R, Russell R, Rutgeerts P, Sanderson J, Sans M, Schumm P, Seibold F, Sharma Y, Simms LA, Seielstad M, Steinhart AH, Targan SR, van den Berg LH, Vatn M, Verspaget H, Walters T, Wijmenga C, Wilson DC, Westra HJ, Xavier RJ, Zhao ZZ, Ponsioen CY, Andersen V, Torkvist L, Gazouli M, Anagnou NP, Karlsen TH, Kupcinskas L, Sventoraityte J, Mansfield JC, Kugathasan S, Silverberg MS, Halfvarson J, Rotter JI, Mathew CG, Griffiths AM, Gearry R, Ahmad T, Brant SR, Chamaillard M, Satsangi J, Cho JH, Schreiber S, Daly MJ, Barrett JC, Parkes M, Annese V, Hakonarson H, Radford-Smith G, Duerr RH, Vermeire S, Weersma RK, Rioux JD. Meta-analysis identifies 29 additional ulcerative colitis risk loci, increasing the number of confirmed associations to 47. Nat Genet 43: 246–252, 2011 - PMC - PubMed
-
- Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, Muir AI, Wigglesworth MJ, Kinghorn I, Fraser NJ, Pike NB, Strum JC, Steplewski KM, Murdock PR, Holder JC, Marshall FH, Szekeres PG, Wilson S, Ignar DM, Foord SM, Wise A, Dowell SJ. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem 278: 11312–11319, 2003 - PubMed
-
- Bu P, Keshavarzian A, Stone DD, Liu J, Le PT, Fisher S, Qiao L. Apoptosis: one of the mechanisms that maintains unresponsiveness of the intestinal mucosal immune system. J Immunol 166: 6399–6403, 2001 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

