Establishment and optimal culture conditions of microrna-induced pluripotent stem cells generated from HEK293 cells via transfection of microrna-302s expression vector
- PMID: 22515122
- PMCID: PMC4831261
Establishment and optimal culture conditions of microrna-induced pluripotent stem cells generated from HEK293 cells via transfection of microrna-302s expression vector
Abstract
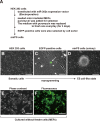
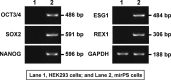
Induced pluripotent stem cells (iPSCs) have been directly generated from fibroblast cultures though retrovirus- or lentivirus-mediated ectopic overexpression of only a few defined transcriptional factors. This remarkable achievement has greatly enhanced our ability to explore the causes of, and potential cures for, many genetic diseases, and strengthened the promise of regenerative medicine. In fact, to date, many kinds of somatic cells from different tissues have exhibited a capacity for reprogramming toward an embryonic stem cell-like state, but major bottlenecks in iPSC derivation and therapeutic use remain, including low reprogramming efficiencies and the tumorigenesis of the generated iPSC. Here, we successfully generated miR-302s-induced pluripotent stem cells (mirPS cells) from human embryonic kidney (HEK) 293 cells via transfection of the miR-302s expression vector. We also determined the optimal culture conditions to generate mirPS on feeder cells, which included the use of serum-free N2B27 medium. The mirPS cells generated by our improved conditions showed the expression of pluripotent marker genes such as OCT3/4, NANOG, and SOX2 under growth conditions via reverse transcription-PCR, whereas no expression of these genes was observed in HEK293 cells. On the other hand, under differentiation conditions, mirPS cells formed ball-shaped structures (embryoid bodies), and showed the ability to differentiate into three germ layers (ectoderm, mesoderm, and endoderm) in vitro. The results suggested that our generated mirPS cells are actually functional as a cell resource to apply to regenerative medicine, and mirPS cells are suitable materials to clarify the mechanism underlying the reprogramming from somatic cells.
Figures

Similar articles
-
Efficient Reprogramming of Canine Peripheral Blood Mononuclear Cells into Induced Pluripotent Stem Cells.Stem Cells Dev. 2021 Jan 15;30(2):79-90. doi: 10.1089/scd.2020.0084. Epub 2020 Dec 24. Stem Cells Dev. 2021. PMID: 33256572
-
Non-integrating episomal plasmid-based reprogramming of human amniotic fluid stem cells into induced pluripotent stem cells in chemically defined conditions.Cell Cycle. 2016;15(2):234-49. doi: 10.1080/15384101.2015.1121332. Cell Cycle. 2016. PMID: 26654216 Free PMC article.
-
Induced pluripotent stem cells from goat fibroblasts.Mol Reprod Dev. 2013 Dec;80(12):1009-17. doi: 10.1002/mrd.22266. Epub 2013 Nov 27. Mol Reprod Dev. 2013. PMID: 24123501
-
Emerging methods for preparing iPS cells.Jpn J Clin Oncol. 2012 Sep;42(9):773-9. doi: 10.1093/jjco/hys108. Epub 2012 Jul 23. Jpn J Clin Oncol. 2012. PMID: 22826352 Review.
-
Choices for Induction of Pluripotency: Recent Developments in Human Induced Pluripotent Stem Cell Reprogramming Strategies.Stem Cell Rev Rep. 2016 Feb;12(1):54-72. doi: 10.1007/s12015-015-9622-8. Stem Cell Rev Rep. 2016. PMID: 26424535 Free PMC article. Review.
Cited by
-
Classical non-homologous end-joining pathway utilizes nascent RNA for error-free double-strand break repair of transcribed genes.Nat Commun. 2016 Oct 5;7:13049. doi: 10.1038/ncomms13049. Nat Commun. 2016. PMID: 27703167 Free PMC article.
-
microRNA control of mouse and human pluripotent stem cell behavior.Annu Rev Cell Dev Biol. 2013;29:213-239. doi: 10.1146/annurev-cellbio-101512-122343. Epub 2013 Jul 12. Annu Rev Cell Dev Biol. 2013. PMID: 23875649 Free PMC article. Review.
-
An Alternate Approach to Generate Induced Pluripotent Stem Cells with Precise CRISPR/Cas9 Tool.Stem Cells Int. 2022 Sep 22;2022:4537335. doi: 10.1155/2022/4537335. eCollection 2022. Stem Cells Int. 2022. PMID: 36187228 Free PMC article.
-
Characterization of embryonic stem-like cells derived from HEK293T cells through miR302/367 expression and their potentiality to differentiate into germ-like cells.Cytotechnology. 2014 Oct;66(5):729-40. doi: 10.1007/s10616-013-9639-2. Epub 2013 Oct 5. Cytotechnology. 2014. PMID: 24091881 Free PMC article.
References
-
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by difined factors. Cell, 2006; 126: 663−676. - PubMed
-
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda H, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by difined factors. Cell, 2007; 131: 861−872. - PubMed
-
- Feng B, Ng JH, Heng JC, Ng HH. Molecules that promote or enhance reprogramming of somatic cells to induced pluripotent stem cells. Cell Stem Cell, 2009; 4: 301−312. - PubMed
-
- Aoi T, Yae K, Nakagawa M, Ichisaka T, Okita K, Takahashi K, Chiba T, Yamanaka S. Generation of pluripotent stem cells from adult mouse liver and stomach cells. Science, 2008; 321: 699−702. - PubMed
-
- Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, Aoi T, Okita K, Mochiduki Y, Takizawa N, Yamanaka S. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol, 2008; 26: 101−106. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
