EYA1 and SIX1 drive the neuronal developmental program in cooperation with the SWI/SNF chromatin-remodeling complex and SOX2 in the mammalian inner ear
- PMID: 22513373
- PMCID: PMC3347689
- DOI: 10.1242/dev.071670
EYA1 and SIX1 drive the neuronal developmental program in cooperation with the SWI/SNF chromatin-remodeling complex and SOX2 in the mammalian inner ear
Abstract
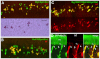
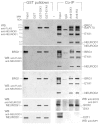
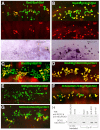
Inner ear neurogenesis depends upon the function of the proneural basic helix-loop-helix (bHLH) transcription factors NEUROG1 and NEUROD1. However, the transcriptional regulation of these factors is unknown. Here, using loss- and gain-of-function models, we show that EYA1 and SIX1 are crucial otic neuronal determination factors upstream of NEUROG1 and NEUROD1. Overexpression of both Eya1 and Six1 is sufficient to convert non-neuronal epithelial cells within the otocyst and cochlea as well as the 3T3 fibroblast cells into neurons. Strikingly, all the ectopic neurons express not only Neurog1 and Neurod1 but also mature neuronal markers such as neurofilament, indicating that Eya1 and Six1 function upstream of, and in the same pathway as, Neurog1 and Neurod1 to not only induce neuronal fate but also regulate their differentiation. We demonstrate that EYA1 and SIX1 interact directly with the SWI/SNF chromatin-remodeling subunits BRG1 and BAF170 to drive neurogenesis cooperatively in 3T3 cells and cochlear nonsensory epithelial cells, and that SOX2 cooperates with these factors to mediate neuronal differentiation. Importantly, we show that the ATPase BRG1 activity is required for not only EYA1- and SIX1-induced ectopic neurogenesis but also normal neurogenesis in the otocyst. These findings indicate that EYA1 and SIX1 are key transcription factors in initiating the neuronal developmental program, probably by recruiting and interacting with the SWI/SNF chromatin-remodeling complex to specifically mediate Neurog1 and Neurod1 transcription.
Figures



Similar articles
-
Eya1-Six1 interaction is sufficient to induce hair cell fate in the cochlea by activating Atoh1 expression in cooperation with Sox2.Dev Cell. 2012 Feb 14;22(2):377-90. doi: 10.1016/j.devcel.2011.12.006. Dev Cell. 2012. PMID: 22340499 Free PMC article.
-
Sox2 induces neuronal formation in the developing mammalian cochlea.J Neurosci. 2010 Jan 13;30(2):714-22. doi: 10.1523/JNEUROSCI.3852-09.2010. J Neurosci. 2010. PMID: 20071536 Free PMC article.
-
Six1 and Eya1 both promote and arrest neuronal differentiation by activating multiple Notch pathway genes.Dev Biol. 2017 Nov 15;431(2):152-167. doi: 10.1016/j.ydbio.2017.09.027. Epub 2017 Sep 22. Dev Biol. 2017. PMID: 28947179
-
Development in the Mammalian Auditory System Depends on Transcription Factors.Int J Mol Sci. 2021 Apr 18;22(8):4189. doi: 10.3390/ijms22084189. Int J Mol Sci. 2021. PMID: 33919542 Free PMC article. Review.
-
EYA1-SIX1 complex in neurosensory cell fate induction in the mammalian inner ear.Hear Res. 2013 Mar;297:13-9. doi: 10.1016/j.heares.2012.09.009. Epub 2012 Oct 24. Hear Res. 2013. PMID: 23104013 Free PMC article. Review.
Cited by
-
The role of Atonal transcription factors in the development of mechanosensitive cells.Semin Cell Dev Biol. 2013 May;24(5):438-47. doi: 10.1016/j.semcdb.2013.03.010. Epub 2013 Mar 30. Semin Cell Dev Biol. 2013. PMID: 23548731 Free PMC article. Review.
-
Making connections in the inner ear: recent insights into the development of spiral ganglion neurons and their connectivity with sensory hair cells.Semin Cell Dev Biol. 2013 May;24(5):460-9. doi: 10.1016/j.semcdb.2013.04.003. Epub 2013 May 6. Semin Cell Dev Biol. 2013. PMID: 23660234 Free PMC article. Review.
-
The canonical wnt signal restricts the glycogen synthase kinase 3/fbw7-dependent ubiquitination and degradation of eya1 phosphatase.Mol Cell Biol. 2014 Jul;34(13):2409-17. doi: 10.1128/MCB.00104-14. Epub 2014 Apr 21. Mol Cell Biol. 2014. PMID: 24752894 Free PMC article.
-
Master regulators in development: Views from the Drosophila retinal determination and mammalian pluripotency gene networks.Dev Biol. 2017 Jan 15;421(2):93-107. doi: 10.1016/j.ydbio.2016.12.005. Epub 2016 Dec 13. Dev Biol. 2017. PMID: 27979656 Free PMC article. Review.
-
Gene, cell, and organ multiplication drives inner ear evolution.Dev Biol. 2017 Nov 1;431(1):3-15. doi: 10.1016/j.ydbio.2017.08.034. Epub 2017 Sep 1. Dev Biol. 2017. PMID: 28866362 Free PMC article. Review.
References
-
- Abdelhak S., Kalatzis V., Heilig R., Compain S., Samson D., Vincent C., Weil D., Cruaud C., Sahly I., Leibovici M., et al. (1997). A human homologue of the Drosophila eyes absent gene underlies branchio-oto-renal (BOR) syndrome and identifies a novel gene family. Nat. Genet. 15, 157–164 - PubMed
-
- Borsani G., DeGrandi A., Ballabio A., Bulfone A., Bernard L., Banfi S., Gattuso C., Mariani M., Dixon M., Donnai D., et al. (1999). EYA4, a novel vertebrate gene related to Drosophila eyes absent. Hum. Mol. Genet. 8, 11–23 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

