Multiple repeat elements within the FAM21 tail link the WASH actin regulatory complex to the retromer
- PMID: 22513087
- PMCID: PMC3374753
- DOI: 10.1091/mbc.E11-12-1059
Multiple repeat elements within the FAM21 tail link the WASH actin regulatory complex to the retromer
Abstract
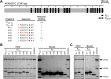
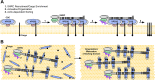
Wiskott-Aldrich syndrome protein (WASPs) control actin dynamics in cellular processes, including cell motility, receptor-mediated endocytosis, bacterial invasion, and vesicular trafficking. We demonstrated that WASH, a recently identified WASP family protein, colocalizes on endosomal subdomains with the cargo-selective complex (CSC) of the retromer, where it regulates retrograde sorting from endosomes in an actin-dependent manner. However, the mechanism of WASH recruitment to these retromer-enriched endosomal subdomains is unclear. Here we show that a component of the WASH regulatory complex (SHRC), FAM21, which contains 21 copies of a novel L-F-[D/E](3-10)-L-F motif, directly interacts with the retromer CSC protein VPS35. Endosomal localization of FAM21 is VPS35 dependent and relies on multivalency of FAM21 repeat elements. Using a combination of pull-down assays and isothermal calorimetry, we demonstrate that individual repeats can bind CSC, and binding affinity varies among different FAM21 repeats. A high-affinity repeat can be converted into a low-affinity one by mutation of a hydrophobic residue within the motif. These in vitro data mirror the localization of FAM21 to retromer-coated vesicles in cells. We propose that multivalency enables FAM21 to sense the density of retromer on membranes, allowing coordination of SHRC recruitment, and consequent actin polymerization, with retromer sorting domain organization/maturation.
Figures

Similar articles
-
Endosomal recruitment of the WASH complex: active sequences and mutations impairing interaction with the retromer.Biol Cell. 2013 May;105(5):191-207. doi: 10.1111/boc.201200038. Epub 2013 Mar 7. Biol Cell. 2013. PMID: 23331060
-
Recruitment of the endosomal WASH complex is mediated by the extended 'tail' of Fam21 binding to the retromer protein Vps35.Biochem J. 2012 Feb 15;442(1):209-20. doi: 10.1042/BJ20111761. Biochem J. 2012. PMID: 22070227
-
Structural basis for coupling of the WASH subunit FAM21 with the endosomal SNX27-Retromer complex.Proc Natl Acad Sci U S A. 2024 Aug 13;121(33):e2405041121. doi: 10.1073/pnas.2405041121. Epub 2024 Aug 8. Proc Natl Acad Sci U S A. 2024. PMID: 39116126 Free PMC article.
-
Retromer-mediated endosomal protein sorting: all WASHed up!Trends Cell Biol. 2013 Nov;23(11):522-8. doi: 10.1016/j.tcb.2013.04.010. Epub 2013 May 28. Trends Cell Biol. 2013. PMID: 23721880 Free PMC article. Review.
-
Actin-dependent endosomal receptor recycling.Curr Opin Cell Biol. 2019 Feb;56:22-33. doi: 10.1016/j.ceb.2018.08.006. Epub 2018 Sep 15. Curr Opin Cell Biol. 2019. PMID: 30227382 Review.
Cited by
-
VARP is recruited on to endosomes by direct interaction with retromer, where together they function in export to the cell surface.Dev Cell. 2014 Jun 9;29(5):591-606. doi: 10.1016/j.devcel.2014.04.010. Epub 2014 May 22. Dev Cell. 2014. PMID: 24856514 Free PMC article.
-
Structural Organization of the Retriever-CCC Endosomal Recycling Complex.bioRxiv [Preprint]. 2023 Jun 7:2023.06.06.543888. doi: 10.1101/2023.06.06.543888. bioRxiv. 2023. Update in: Nat Struct Mol Biol. 2024 Jun;31(6):910-924. doi: 10.1038/s41594-023-01184-4. PMID: 37333304 Free PMC article. Updated. Preprint.
-
Structure and interactions of the endogenous human Commander complex.Nat Struct Mol Biol. 2024 Jun;31(6):925-938. doi: 10.1038/s41594-024-01246-1. Epub 2024 Mar 8. Nat Struct Mol Biol. 2024. PMID: 38459129 Free PMC article.
-
CCC- and WASH-mediated endosomal sorting of LDLR is required for normal clearance of circulating LDL.Nat Commun. 2016 Mar 11;7:10961. doi: 10.1038/ncomms10961. Nat Commun. 2016. PMID: 26965651 Free PMC article.
-
Retriever is a multiprotein complex for retromer-independent endosomal cargo recycling.Nat Cell Biol. 2017 Oct;19(10):1214-1225. doi: 10.1038/ncb3610. Epub 2017 Sep 11. Nat Cell Biol. 2017. PMID: 28892079 Free PMC article.
References
-
- Benesch S, Polo S, Lai FP, Anderson KI, Stradal TE, Wehland J, Rottner K. N-WASP deficiency impairs EGF internalization and actin assembly at clathrin-coated pits. J Cell Sci. 2005;118:3103–3115. - PubMed
-
- Bonifacino JS, Traub LM. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Biochem. 2003;72:395–447. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

