Wnt signaling in neuromuscular junction development
- PMID: 22510459
- PMCID: PMC3367558
- DOI: 10.1101/cshperspect.a008045
Wnt signaling in neuromuscular junction development
Abstract
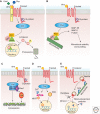
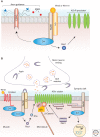
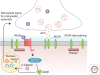
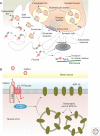
Wnt proteins are best known for their profound roles in cell patterning, because they are required for the embryonic development of all animal species studied to date. Besides regulating cell fate, Wnt proteins are gaining increasing recognition for their roles in nervous system development and function. New studies indicate that multiple positive and negative Wnt signaling pathways take place simultaneously during the formation of vertebrate and invertebrate neuromuscular junctions. Although some Wnts are essential for the formation of NMJs, others appear to play a more modulatory role as part of multiple signaling pathways. Here we review the most recent findings regarding the function of Wnts at the NMJ from both vertebrate and invertebrate model systems.
Figures
Similar articles
-
Dual roles for Wnt signalling during the formation of the vertebrate neuromuscular junction.Acta Physiol (Oxf). 2012 Jan;204(1):128-36. doi: 10.1111/j.1748-1716.2011.02295.x. Epub 2011 May 7. Acta Physiol (Oxf). 2012. PMID: 21554559 Review.
-
The Wnt and BMP families of signaling morphogens at the vertebrate neuromuscular junction.Int J Mol Sci. 2011;12(12):8924-46. doi: 10.3390/ijms12128924. Epub 2011 Dec 5. Int J Mol Sci. 2011. PMID: 22272112 Free PMC article. Review.
-
Wnt proteins contribute to neuromuscular junction formation through distinct signaling pathways.Development. 2017 May 1;144(9):1712-1724. doi: 10.1242/dev.146167. Epub 2017 Mar 27. Development. 2017. PMID: 28348167
-
Crosstalk between Agrin and Wnt signaling pathways in development of vertebrate neuromuscular junction.Dev Neurobiol. 2014 Aug;74(8):828-38. doi: 10.1002/dneu.22190. Epub 2014 Jun 4. Dev Neurobiol. 2014. PMID: 24838312 Review.
-
Analysis of Wnt signaling during Caenorhabditis elegans postembryonic development.Methods Mol Biol. 2008;469:87-102. doi: 10.1007/978-1-60327-469-2_8. Methods Mol Biol. 2008. PMID: 19109705 Review.
Cited by
-
Exosomes go with the Wnt.Cell Logist. 2012 Jul 1;2(3):169-173. doi: 10.4161/cl.21981. Cell Logist. 2012. PMID: 23739155 Free PMC article.
-
Conservation and Innovation: Versatile Roles for LRP4 in Nervous System Development.J Dev Biol. 2021 Mar 14;9(1):9. doi: 10.3390/jdb9010009. J Dev Biol. 2021. PMID: 33799485 Free PMC article. Review.
-
Wnt Signaling in Renal Cell Carcinoma.Cancers (Basel). 2016 Jun 17;8(6):57. doi: 10.3390/cancers8060057. Cancers (Basel). 2016. PMID: 27322325 Free PMC article. Review.
-
Synaptic Homeostasis and Its Immunological Disturbance in Neuromuscular Junction Disorders.Int J Mol Sci. 2017 Apr 24;18(4):896. doi: 10.3390/ijms18040896. Int J Mol Sci. 2017. PMID: 28441759 Free PMC article. Review.
-
Molecular Chaperone Calnexin Regulates the Function of Drosophila Sodium Channel Paralytic.Front Mol Neurosci. 2017 Mar 7;10:57. doi: 10.3389/fnmol.2017.00057. eCollection 2017. Front Mol Neurosci. 2017. PMID: 28326013 Free PMC article.
References
-
- Aoki K, Taketo MM 2007. Adenomatous polyposis coli (APC): A multi-functional tumor suppressor gene. J Cell Sci 120: 3327–3335 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
