Role of miR-148a in hepatitis B associated hepatocellular carcinoma
- PMID: 22496917
- PMCID: PMC3322146
- DOI: 10.1371/journal.pone.0035331
Role of miR-148a in hepatitis B associated hepatocellular carcinoma
Abstract
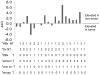
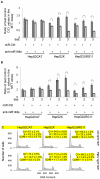
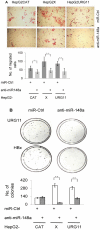
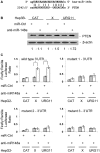
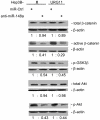
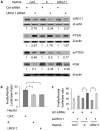
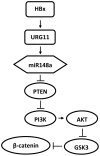
Hepatitis B virus encoded X antigen (HBx) is a trans-regulatory protein that alters the activity of selected transcription factors and cytoplasmic signal transduction pathways. HBx transcriptionally up-regulates the expression of a unique gene, URG11, which in turn transcriptionally up-regulates β-catenin, thereby contributing importantly to hepatocarcinogenesis. HBx and URG11 also alter the expression of multiple microRNAs, and by miRNA array analysis, both were shown to promote the expression of miR-148a. Elevated miR-148a was also seen in HBx positive liver samples from infected patients. To study the function of miR-148a, anti-148a was introduced into HepG2 and Hep3B cells stably expressing HBx or stably over-expressing URG11. Anti-miR-148a suppressed cell proliferation, cell cycle progression, cell migration, anchorage independent growth in soft agar and subcutaneous tumor formation in SCID mice. Introduction of anti-miR-148a increased PTEN protein and mRNA expression, suggesting that PTEN was targeted by miR-148a. Anti-miR-148a failed to suppress PTEN expression when co-transfected with reporter gene mutants in the 3'UTR of PTEN mRNA. Introduction of anti-miR-148a also resulted in depressed Akt signaling by HBx and URG11, resulting in decreased expression of β-catenin. Thus, miR-148a may play a central role in HBx/URG11 mediated HCC, and may be an early diagnostic marker and/or therapeutic target associated with this tumor type.
Conflict of interest statement
Figures

Similar articles
-
Upregulated microRNA-29a by hepatitis B virus X protein enhances hepatoma cell migration by targeting PTEN in cell culture model.PLoS One. 2011 May 5;6(5):e19518. doi: 10.1371/journal.pone.0019518. PLoS One. 2011. PMID: 21573166 Free PMC article.
-
MiR-19a, miR-122 and miR-223 are differentially regulated by hepatitis B virus X protein and involve in cell proliferation in hepatoma cells.J Transl Med. 2016 May 5;14(1):122. doi: 10.1186/s12967-016-0888-7. J Transl Med. 2016. PMID: 27150195 Free PMC article.
-
Hepatitis B virus X protein represses miRNA-148a to enhance tumorigenesis.J Clin Invest. 2013 Feb;123(2):630-45. doi: 10.1172/JCI64265. Epub 2013 Jan 16. J Clin Invest. 2013. PMID: 23321675 Free PMC article.
-
Involvement of cholesterol in hepatitis B virus X protein-induced abnormal lipid metabolism of hepatoma cells via up-regulating miR-205-targeted ACSL4.Biochem Biophys Res Commun. 2014 Mar 14;445(3):651-5. doi: 10.1016/j.bbrc.2014.02.068. Epub 2014 Feb 24. Biochem Biophys Res Commun. 2014. PMID: 24576478
-
The Epigenetic Modulation of Cancer and Immune Pathways in Hepatitis B Virus-Associated Hepatocellular Carcinoma: The Influence of HBx and miRNA Dysregulation.Front Immunol. 2021 Apr 29;12:661204. doi: 10.3389/fimmu.2021.661204. eCollection 2021. Front Immunol. 2021. PMID: 33995383 Free PMC article. Review.
Cited by
-
Dysregulated microRNAs as a biomarker for diagnosis and prognosis of hepatitis B virus-associated hepatocellular carcinoma.World J Gastroenterol. 2023 Aug 21;29(31):4706-4735. doi: 10.3748/wjg.v29.i31.4706. World J Gastroenterol. 2023. PMID: 37664153 Free PMC article. Review.
-
Hepatitis B virus induces cell proliferation via HBx-induced microRNA-21 in hepatocellular carcinoma by targeting programmed cell death protein4 (PDCD4) and phosphatase and tensin homologue (PTEN).PLoS One. 2014 Mar 14;9(3):e91745. doi: 10.1371/journal.pone.0091745. eCollection 2014. PLoS One. 2014. PMID: 24633222 Free PMC article.
-
Implications of biomarkers in human hepatocellular carcinoma pathogenesis and therapy.World J Gastroenterol. 2014 Aug 14;20(30):10249-61. doi: 10.3748/wjg.v20.i30.10249. World J Gastroenterol. 2014. PMID: 25132742 Free PMC article. Review.
-
Development of MicroRNA Therapeutics for Hepatocellular Carcinoma.Diagnostics (Basel). 2013 Mar 15;3(1):170-91. doi: 10.3390/diagnostics3010170. Diagnostics (Basel). 2013. PMID: 26835673 Free PMC article. Review.
-
MicroRNA-548a-5p promotes proliferation and inhibits apoptosis in hepatocellular carcinoma cells by targeting Tg737.World J Gastroenterol. 2016 Jun 21;22(23):5364-73. doi: 10.3748/wjg.v22.i23.5364. World J Gastroenterol. 2016. PMID: 27340352 Free PMC article.
References
-
- Tiollais P, Pourcel C, Dejean A. The hepatitis B virus. Nature. 1985;317:489–495. - PubMed
-
- Szmuness W. Hepatocellular carcinoma and the hepatitis B virus: evidence for a causal association. Prog Med Virol. 1978;24:40–69. - PubMed
-
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. - PubMed
-
- Henkler FF, Koshy R. Hepatitis B virus transcriptional activators: mechanisms and possible role in oncogenesis. J Viral Hepat. 1996;3:109–121. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

