Modification of Ad5 hexon hypervariable regions circumvents pre-existing Ad5 neutralizing antibodies and induces protective immune responses
- PMID: 22496772
- PMCID: PMC3320611
- DOI: 10.1371/journal.pone.0033920
Modification of Ad5 hexon hypervariable regions circumvents pre-existing Ad5 neutralizing antibodies and induces protective immune responses
Erratum in
- PLoS One. 2012;7(5): doi/10.1371/annotation/c110beed-3cac-48db-9039-ba4498d5db50
Abstract
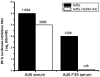
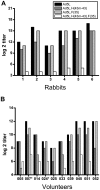
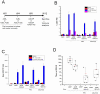
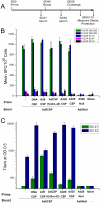
The development of an effective malaria vaccine is a high global health priority. Vaccine vectors based on adenovirus type 5 are capable of generating robust and protective T cell and antibody responses in animal models and are currently being evaluated in clinical trials for HIV and malaria. They appear to be more effective in terms of inducing antigen-specific immune responses as compared with non-Ad5 serotype vectors. However, the high prevalence of neutralizing antibodies to Ad5 in the human population, particularly in the developing world, has the potential to limit the effectiveness of Ad5-based vaccines. We have generated novel Ad5-based vectors that precisely replace the hexon hypervariable regions with those derived from Ad43, a subgroup D serotype with low prevalence of neutralizing antibody in humans. We have demonstrated that these hexon-modified adenovectors are not neutralized efficiently by Ad5 neutralizing antibodies in vitro using sera from mice, rabbits and human volunteers. We have also generated hexon-modified adenovectors that express a rodent malaria parasite antigen, PyCSP, and demonstrated that they are as immunogenic as an unmodified vector. Furthermore, in contrast to the unmodified vector, the hexon-modified adenovectors induced robust T cell responses in mice with high levels of Ad5 neutralizing antibody. We also show that the hexon-modified vector can be combined with unmodified Ad5 vector in prime-boost regimens to induce protective responses in mice. Our data establish that these hexon-modified vectors are highly immunogenic even in the presence of pre-existing anti-adenovirus antibodies. These hexon-modified adenovectors may have advantages in sub-Saharan Africa where there is a high prevalence of Ad5 neutralizing antibody in the population.
Conflict of interest statement
Figures



Similar articles
-
Identification of a suppressor mutation that improves the yields of hexon-modified adenovirus vectors.J Virol. 2013 Sep;87(17):9661-71. doi: 10.1128/JVI.00462-13. Epub 2013 Jul 3. J Virol. 2013. PMID: 23824800 Free PMC article.
-
Hexon-chimaeric adenovirus serotype 5 vectors circumvent pre-existing anti-vector immunity.Nature. 2006 May 11;441(7090):239-43. doi: 10.1038/nature04721. Epub 2006 Apr 16. Nature. 2006. PMID: 16625206
-
Neutralizing antibodies to adenovirus serotype 5 vaccine vectors are directed primarily against the adenovirus hexon protein.J Immunol. 2005 Jun 1;174(11):7179-85. doi: 10.4049/jimmunol.174.11.7179. J Immunol. 2005. PMID: 15905562
-
Hexon hypervariable region-modified adenovirus type 5 (Ad5) vectors display reduced hepatotoxicity but induce T lymphocyte phenotypes similar to Ad5 vectors.Clin Vaccine Immunol. 2014 Aug;21(8):1137-44. doi: 10.1128/CVI.00207-14. Epub 2014 Jun 18. Clin Vaccine Immunol. 2014. PMID: 24943382 Free PMC article.
-
Overcoming pre-existing adenovirus immunity by genetic engineering of adenovirus-based vectors.Expert Opin Biol Ther. 2009 Dec;9(12):1521-31. doi: 10.1517/14712590903307388. Expert Opin Biol Ther. 2009. PMID: 19780714 Review.
Cited by
-
Diverse genotypes of human enteric and non-enteric adenoviruses circulating in children hospitalized with acute gastroenteritis in Thailand, from 2018 to 2021.Microbiol Spectr. 2023 Aug 17;11(5):e0117323. doi: 10.1128/spectrum.01173-23. Online ahead of print. Microbiol Spectr. 2023. PMID: 37589466 Free PMC article.
-
Circumventing antivector immunity: potential use of nonhuman adenoviral vectors.Hum Gene Ther. 2014 Apr;25(4):285-300. doi: 10.1089/hum.2013.228. Epub 2014 Mar 25. Hum Gene Ther. 2014. PMID: 24499174 Free PMC article. Review.
-
Identification of a suppressor mutation that improves the yields of hexon-modified adenovirus vectors.J Virol. 2013 Sep;87(17):9661-71. doi: 10.1128/JVI.00462-13. Epub 2013 Jul 3. J Virol. 2013. PMID: 23824800 Free PMC article.
-
Adenovirus with DNA Packaging Gene Mutations Increased Virus Release.Viruses. 2016 Dec 20;8(12):333. doi: 10.3390/v8120333. Viruses. 2016. PMID: 27999391 Free PMC article.
-
The march toward malaria vaccines.Vaccine. 2015 Nov 27;33 Suppl 4(Suppl 4):D13-23. doi: 10.1016/j.vaccine.2015.07.091. Epub 2015 Aug 29. Vaccine. 2015. PMID: 26324116 Free PMC article. Review.
References
-
- Rasmussen H, Rasmussen C, Lempicki M, Durham R, Brough D, et al. TNFerade Biologic: preclinical toxicology of a novel adenovector with a radiation-inducible promoter, carrying the human tumor necrosis factor alpha gene. Cancer Gene Ther. 2002;9:951–957. - PubMed
-
- Shiver JW, Emini EA. Recent advances in the development of HIV-1 vaccines using replication-incompetent adenovirus vectors. Annu Rev Med. 2004;55:355–372. - PubMed
-
- Bruder JT, Angove E, Limbach KJ, Richie TL. Molecular Vaccines for Malaria. Human Vaccines. 2010;6:1:1–25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

