Exploitation of evolutionarily conserved amoeba and mammalian processes by Legionella
- PMID: 22494803
- PMCID: PMC3603140
- DOI: 10.1016/j.tim.2012.03.005
Exploitation of evolutionarily conserved amoeba and mammalian processes by Legionella
Abstract
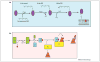
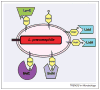
Legionella pneumophila proliferates within various protists and metazoan cells, where a cadre of ∼300 effectors is injected into the host cell by the defect in organelle trafficking/intracellular multiplication (Dot/Icm) type IVB translocation system. Interkingdom horizontal gene transfer of genes of protists and their subsequent convergent evolution to become translocated effectors has probably enabled L. pneumophila to adapt to the intracellular life within various protists and metazoan cells through exploitation of evolutionarily eukaryotic processes, such as endoplasmic reticulum-to-Golgi vesicle traffic, phosphoinositol metabolism, AMPylation, deAMPylation, prenylation, polyubiquitination, proteasomal degradation and cytosolic amino- and oligo-peptidases. This is highlighted by the ankyrin B (AnkB) F-box effector that exploits multiple conserved eukaryotic machineries to generate high levels of free amino acids as sources of carbon and energy essential for intracellular proliferation in protists and metazoan cells and for manifestation of pulmonary disease in mammals.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

Similar articles
-
Cellular microbiology and molecular ecology of Legionella-amoeba interaction.Virulence. 2013 May 15;4(4):307-14. doi: 10.4161/viru.24290. Epub 2013 Mar 27. Virulence. 2013. PMID: 23535283 Free PMC article. Review.
-
Evolution and Adaptation of Legionella pneumophila to Manipulate the Ubiquitination Machinery of Its Amoebae and Mammalian Hosts.Biomolecules. 2021 Jan 15;11(1):112. doi: 10.3390/biom11010112. Biomolecules. 2021. PMID: 33467718 Free PMC article. Review.
-
Molecular mimicry by an F-box effector of Legionella pneumophila hijacks a conserved polyubiquitination machinery within macrophages and protozoa.PLoS Pathog. 2009 Dec;5(12):e1000704. doi: 10.1371/journal.ppat.1000704. Epub 2009 Dec 24. PLoS Pathog. 2009. PMID: 20041211 Free PMC article.
-
Molecular Characterization of Exploitation of the Polyubiquitination and Farnesylation Machineries of Dictyostelium Discoideum by the AnkB F-Box Effector of Legionella Pneumophila.Front Microbiol. 2011 Feb 14;2:23. doi: 10.3389/fmicb.2011.00023. eCollection 2011. Front Microbiol. 2011. PMID: 21687415 Free PMC article.
-
Exploitation of conserved eukaryotic host cell farnesylation machinery by an F-box effector of Legionella pneumophila.J Exp Med. 2010 Aug 2;207(8):1713-26. doi: 10.1084/jem.20100771. Epub 2010 Jul 26. J Exp Med. 2010. PMID: 20660614 Free PMC article.
Cited by
-
Ciliate Paramecium is a natural reservoir of Legionella pneumophila.Sci Rep. 2016 Apr 15;6:24322. doi: 10.1038/srep24322. Sci Rep. 2016. PMID: 27079173 Free PMC article.
-
Protein sociology of ProA, Mip and other secreted virulence factors at the Legionella pneumophila surface.Front Cell Infect Microbiol. 2023 Mar 2;13:1140688. doi: 10.3389/fcimb.2023.1140688. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 36936764 Free PMC article. Review.
-
Rapid nutritional remodeling of the host cell upon attachment of Legionella pneumophila.Infect Immun. 2014 Jan;82(1):72-82. doi: 10.1128/IAI.01079-13. Epub 2013 Oct 14. Infect Immun. 2014. PMID: 24126522 Free PMC article.
-
Complete and ubiquitinated proteome of the Legionella-containing vacuole within human macrophages.J Proteome Res. 2015 Jan 2;14(1):236-48. doi: 10.1021/pr500765x. Epub 2014 Nov 13. J Proteome Res. 2015. PMID: 25369898 Free PMC article.
-
Amoebae in Chronic, Polymicrobial Endodontic Infections Are Associated with Altered Microbial Communities of Increased Virulence.J Clin Med. 2020 Nov 18;9(11):3700. doi: 10.3390/jcm9113700. J Clin Med. 2020. PMID: 33218015 Free PMC article.
References
-
- Harb OS, et al. From protozoa to mammalian cells: a new paradigm in the life cycle of intracellular bacterial pathogens. Environ Microbiol. 2000;2:251–265. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

