Differential estrogenic actions of endocrine-disrupting chemicals bisphenol A, bisphenol AF, and zearalenone through estrogen receptor α and β in vitro
- PMID: 22494775
- PMCID: PMC3404668
- DOI: 10.1289/ehp.1104689
Differential estrogenic actions of endocrine-disrupting chemicals bisphenol A, bisphenol AF, and zearalenone through estrogen receptor α and β in vitro
Erratum in
- Environ Health Perspect. 2012 Dec;120(12):A455
Abstract
Background: Endocrine-disrupting chemicals (EDCs) are widely found in the environment. Estrogen-like activity is attributed to EDCs, such as bisphenol A (BPA), bisphenol AF (BPAF), and zearalenone (Zea), but mechanisms of action and diversity of effects are poorly understood.
Objectives: We used in vitro models to evaluate the mechanistic actions of BPA, BPAF, and Zea on estrogen receptor (ER) α and ERβ.
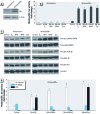
Methods: We used three human cell lines (Ishikawa, HeLa, and HepG2) representing three cell types to evaluate the estrogen promoter activity of BPA, BPAF, and Zea on ERα and ERβ. Ishikawa/ERα stable cells were used to determine changes in estrogen response element (ERE)-mediated target gene expression or rapid action-mediated effects.
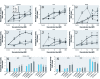
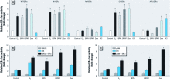
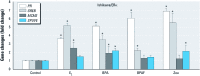
Results: The three EDCs showed strong estrogenic activity as agonists for ERα in a dose-dependent manner. At lower concentrations, BPA acted as an antagonist for ERα in Ishikawa cells and BPAF acted as an antagonist for ERβ in HeLa cells, whereas Zea was only a partial antagonist for ERα. ERE-mediated activation by BPA and BPAF was via the AF-2 function of ERα, but Zea activated via both the AF-1 and AF-2 functions. Endogenous ERα target genes and rapid signaling via the p44/42 MAPK pathway were activated by BPA, BPAF, and Zea.
Conclusion: BPA and BPAF can function as EDCs by acting as cell type-specific agonists (≥ 10 nM) or antagonists (≤ 10 nM) for ERα and ERβ. Zea had strong estrogenic activity and activated both the AF-1 and AF-2 functions of ERα. In addition, all three compounds induced the rapid action-mediated response for ERα.
Conflict of interest statement
The authors declare they have no actual or potential competing financial interests.
Figures
Similar articles
-
Differential in Vitro Biological Action, Coregulator Interactions, and Molecular Dynamic Analysis of Bisphenol A (BPA), BPAF, and BPS Ligand-ERα Complexes.Environ Health Perspect. 2018 Jan 31;126(1):017012. doi: 10.1289/EHP2505. Environ Health Perspect. 2018. PMID: 29389661 Free PMC article.
-
Endocrine-Disrupting Chemicals (EDCs): In Vitro Mechanism of Estrogenic Activation and Differential Effects on ER Target Genes.Environ Health Perspect. 2013 Apr;121(4):459-66. doi: 10.1289/ehp.1205951. Epub 2013 Feb 5. Environ Health Perspect. 2013. PMID: 23384675 Free PMC article.
-
Bisphenol AF as an Inducer of Estrogen Receptor β (ERβ): Evidence for Anti-estrogenic Effects at Higher Concentrations in Human Breast Cancer Cells.Biol Pharm Bull. 2017;40(11):1909-1916. doi: 10.1248/bpb.b17-00427. Biol Pharm Bull. 2017. PMID: 29093337
-
Estrogen Receptor β as a Possible Double-Edged Sword Molecule in Breast Cancer: A Mechanism of Alteration of Its Role by Exposure to Endocrine-Disrupting Chemicals.Biol Pharm Bull. 2021;44(11):1594-1597. doi: 10.1248/bpb.b21-00468. Biol Pharm Bull. 2021. PMID: 34719637 Review.
-
Extranuclear-initiated estrogenic actions of endocrine disrupting chemicals: Is there toxicology beyond paracelsus?J Steroid Biochem Mol Biol. 2018 Feb;176:16-22. doi: 10.1016/j.jsbmb.2017.01.014. Epub 2017 Jan 31. J Steroid Biochem Mol Biol. 2018. PMID: 28159674 Review.
Cited by
-
The Inhibitory Potential of Selected Essential Oils on Fusarium spp. Growth and Mycotoxins Biosynthesis in Maize Seeds.Pathogens. 2019 Dec 26;9(1):23. doi: 10.3390/pathogens9010023. Pathogens. 2019. PMID: 31887989 Free PMC article.
-
Dietary exposure to mycotoxin zearalenone (ZEA) during post-implantation adversely affects placental development in mice.Reprod Toxicol. 2019 Apr;85:42-50. doi: 10.1016/j.reprotox.2019.01.010. Epub 2019 Feb 14. Reprod Toxicol. 2019. PMID: 30772436 Free PMC article.
-
Genome-Wide Analysis of Low Dose Bisphenol-A (BPA) Exposure in Human Prostate Cells.Curr Genomics. 2019 May;20(4):260-274. doi: 10.2174/1389202920666190603123040. Curr Genomics. 2019. PMID: 32030086 Free PMC article.
-
Effect of bisphenol a on occurrence and progression of prolactinoma and its underlying mechanisms.Am J Transl Res. 2016 Oct 15;8(10):4195-4204. eCollection 2016. Am J Transl Res. 2016. PMID: 27830003 Free PMC article.
-
Endocrine Disrupting Chemicals and Endometrial Cancer: An Overview of Recent Laboratory Evidence and Epidemiological Studies.Int J Environ Res Public Health. 2017 Mar 22;14(3):334. doi: 10.3390/ijerph14030334. Int J Environ Res Public Health. 2017. PMID: 28327540 Free PMC article. Review.
References
-
- Akahori Y, Nakai M, Yamasaki K, Takatsuki M, Shimohigashi Y, Ohtaki M. Relationship between the results of in vitro receptor binding assay to human estrogen receptor α and in vivo uterotrophic assay: comparative study with 65 selected chemicals. Toxicol in Vitro. 2008;22:225–231. - PubMed
-
- Bay K, Andersson AM, Skakkebaek NE. Estradiol levels in prepubertal boys and girls–analytical challenges. Int J Androl. 2004;27:266–273. - PubMed
-
- Boehme K, Simon S, Mueller SO. Gene expression profiling in Ishikawa cells: a fingerprint for estrogen active compounds. Toxicol Appl Pharmacol. 2009;236:85–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

