Proinflammatory cytokine signaling required for the generation of natural killer cell memory
- PMID: 22493516
- PMCID: PMC3348098
- DOI: 10.1084/jem.20111760
Proinflammatory cytokine signaling required for the generation of natural killer cell memory
Abstract
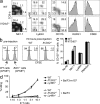
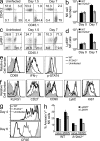
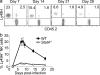
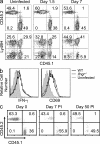
Although natural killer (NK) cells are classified as innate immune cells, recent studies demonstrate that NK cells can become long-lived memory cells and contribute to secondary immune responses. The precise signals that promote generation of long-lived memory NK cells are unknown. Using cytokine receptor-deficient mice, we show that interleukin-12 (IL-12) is indispensible for mouse cytomegalovirus (MCMV)-specific NK cell expansion and generation of memory NK cells. In contrast to wild-type NK cells that proliferated robustly and resided in lymphoid and nonlymphoid tissues for months after MCMV infection, IL-12 receptor-deficient NK cells failed to expand and were unable to mediate protection after MCMV challenge. We further demonstrate that a STAT4-dependent IFN-γ-independent mechanism contributes toward the generation of memory NK cells during MCMV infection. Understanding the full contribution of inflammatory cytokine signaling to the NK cell response against viral infection will be of interest for the development of vaccines and therapeutics.
Figures


Similar articles
-
Cell-intrinsic adrenergic signaling controls the adaptive NK cell response to viral infection.J Exp Med. 2020 Apr 6;217(4):e20190549. doi: 10.1084/jem.20190549. J Exp Med. 2020. PMID: 32045471 Free PMC article.
-
Stage-specific regulation of natural killer cell homeostasis and response against viral infection by microRNA-155.Proc Natl Acad Sci U S A. 2013 Apr 23;110(17):6967-72. doi: 10.1073/pnas.1304410110. Epub 2013 Apr 9. Proc Natl Acad Sci U S A. 2013. PMID: 23572582 Free PMC article.
-
Cytomegalovirus generates long-lived antigen-specific NK cells with diminished bystander activation to heterologous infection.J Exp Med. 2014 Dec 15;211(13):2669-80. doi: 10.1084/jem.20141172. Epub 2014 Nov 24. J Exp Med. 2014. PMID: 25422494 Free PMC article.
-
Molecular Programming of Immunological Memory in Natural Killer Cells.Adv Exp Med Biol. 2015;850:81-91. doi: 10.1007/978-3-319-15774-0_7. Adv Exp Med Biol. 2015. PMID: 26324348 Review.
-
Immunoregulatory cytokine networks: 60 years of learning from murine cytomegalovirus.Med Microbiol Immunol. 2015 Jun;204(3):345-54. doi: 10.1007/s00430-015-0412-3. Epub 2015 Apr 8. Med Microbiol Immunol. 2015. PMID: 25850988 Free PMC article. Review.
Cited by
-
Distinguishing rheumatoid arthritis from psoriatic arthritis.RMD Open. 2018 Aug 13;4(2):e000656. doi: 10.1136/rmdopen-2018-000656. eCollection 2018. RMD Open. 2018. PMID: 30167326 Free PMC article.
-
Natural Killer Cells Adapt to Cytomegalovirus Along a Functionally Static Phenotypic Spectrum in Human Immunodeficiency Virus Infection.Front Immunol. 2018 Nov 12;9:2494. doi: 10.3389/fimmu.2018.02494. eCollection 2018. Front Immunol. 2018. PMID: 30483249 Free PMC article.
-
Trained Immunity Carried by Non-immune Cells.Front Microbiol. 2019 Jan 14;9:3225. doi: 10.3389/fmicb.2018.03225. eCollection 2018. Front Microbiol. 2019. PMID: 30692968 Free PMC article. Review.
-
Intravital Imaging of Inflammatory Response in Liver Disease.Front Cell Dev Biol. 2022 Jun 28;10:922041. doi: 10.3389/fcell.2022.922041. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35837329 Free PMC article. Review.
-
Immunoglobulin-like receptors and the generation of innate immune memory.Immunogenetics. 2022 Feb;74(1):179-195. doi: 10.1007/s00251-021-01240-7. Epub 2022 Jan 16. Immunogenetics. 2022. PMID: 35034136 Free PMC article. Review.
References
-
- Björkström N.K., Lindgren T., Stoltz M., Fauriat C., Braun M., Evander M., Michaëlsson J., Malmberg K.J., Klingström J., Ahlm C., Ljunggren H.G. 2011. Rapid expansion and long-term persistence of elevated NK cell numbers in humans infected with hantavirus. J. Exp. Med. 208:13–21 10.1084/jem.20100762 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

