Kruppel-like factor 15 (KLF15) is a key regulator of podocyte differentiation
- PMID: 22493483
- PMCID: PMC3365945
- DOI: 10.1074/jbc.M112.345983
Kruppel-like factor 15 (KLF15) is a key regulator of podocyte differentiation
Abstract
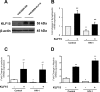
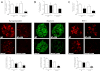
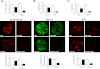
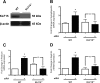
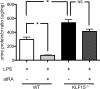
Podocyte injury resulting from a loss of differentiation is the hallmark of many glomerular diseases. We previously showed that retinoic acid (RA) induces podocyte differentiation via stimulation of the cAMP pathway. However, many podocyte maturity markers lack binding sites for RA-response element or cAMP-response element (CREB) in their promoter regions. We hypothesized that transcription factors induced by RA and downstream of CREB mediate podocyte differentiation. We performed microarray gene expression studies in human podocytes treated with and without RA to identify differentially regulated genes. In comparison with known CREB target genes, we identified Krüppel-like factor 15 (KLF15), a kidney-enriched nuclear transcription factor, that has been previously shown to mediate cell differentiation. We confirmed that RA increased KLF15 expression in both murine and human podocytes. Overexpression of KLF15 stimulated expression of differentiation markers in both wild-type and HIV-1-infected podocytes. Also, KLF15 binding to the promoter regions of nephrin and podocin was increased in RA-treated podocytes. Although KLF15(-/-) mice at base line had minimal phenotype, lipopolysaccharide- or adriamycin-treated KLF15(-/-) mice had a significant increase in proteinuria and podocyte foot process effacement with a reduction in the expression of podocyte differentiation markers as compared with the wild-type treated mice. Finally, KLF15 expression was reduced in glomeruli isolated from HIV transgenic mice as well as in kidney biopsies from patients with HIV-associated nephropathy and idiopathic focal segmental glomerulosclerosis. These results indicate a critical role of KLF15 in mediating podocyte differentiation and in protecting podocytes against injury.
Figures
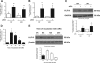

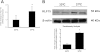


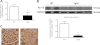

Similar articles
-
Krüppel-Like Factor 15 Mediates Glucocorticoid-Induced Restoration of Podocyte Differentiation Markers.J Am Soc Nephrol. 2017 Jan;28(1):166-184. doi: 10.1681/ASN.2015060672. Epub 2016 Jun 10. J Am Soc Nephrol. 2017. PMID: 27288011 Free PMC article.
-
Podocyte-Specific Induction of Krüppel-Like Factor 15 Restores Differentiation Markers and Attenuates Kidney Injury in Proteinuric Kidney Disease.J Am Soc Nephrol. 2018 Oct;29(10):2529-2545. doi: 10.1681/ASN.2018030324. Epub 2018 Aug 24. J Am Soc Nephrol. 2018. PMID: 30143559 Free PMC article.
-
The Krüppel-like factor 15-NFATc1 axis ameliorates podocyte injury: a novel rationale for using glucocorticoids in proteinuria diseases.Clin Sci (Lond). 2020 Jun 26;134(12):1305-1318. doi: 10.1042/CS20200075. Clin Sci (Lond). 2020. PMID: 32478397
-
Disparate roles of retinoid acid signaling molecules in kidney disease.Am J Physiol Renal Physiol. 2021 May 1;320(5):F683-F692. doi: 10.1152/ajprenal.00045.2021. Epub 2021 Mar 1. Am J Physiol Renal Physiol. 2021. PMID: 33645319 Free PMC article. Review.
-
Krüppel-like Factor 15: A Potential Therapeutic Target For Kidney Disease.Int J Biol Sci. 2019 Jul 21;15(9):1955-1961. doi: 10.7150/ijbs.34838. eCollection 2019. Int J Biol Sci. 2019. PMID: 31523196 Free PMC article. Review.
Cited by
-
Krüppel-like factor 6 regulates mitochondrial function in the kidney.J Clin Invest. 2015 Mar 2;125(3):1347-61. doi: 10.1172/JCI77084. Epub 2015 Feb 17. J Clin Invest. 2015. PMID: 25689250 Free PMC article.
-
Proteinuria impairs podocyte regeneration by sequestering retinoic acid.J Am Soc Nephrol. 2013 Nov;24(11):1756-68. doi: 10.1681/ASN.2012090950. Epub 2013 Aug 15. J Am Soc Nephrol. 2013. PMID: 23949798 Free PMC article.
-
The controversial role of retinoic acid in fibrotic diseases: analysis of involved signaling pathways.Int J Mol Sci. 2012 Dec 21;14(1):226-43. doi: 10.3390/ijms14010226. Int J Mol Sci. 2012. PMID: 23344030 Free PMC article. Review.
-
Krüppel-Like Factor 15 Mediates Glucocorticoid-Induced Restoration of Podocyte Differentiation Markers.J Am Soc Nephrol. 2017 Jan;28(1):166-184. doi: 10.1681/ASN.2015060672. Epub 2016 Jun 10. J Am Soc Nephrol. 2017. PMID: 27288011 Free PMC article.
-
A KDM6A-KLF10 reinforcing feedback mechanism aggravates diabetic podocyte dysfunction.EMBO Mol Med. 2019 May;11(5):e9828. doi: 10.15252/emmm.201809828. EMBO Mol Med. 2019. PMID: 30948420 Free PMC article.
References
-
- Wolf G., Chen S., Ziyadeh F. N. (2005) From the periphery of the glomerular capillary wall toward the center of disease. Podocyte injury comes of age in diabetic nephropathy. Diabetes 54, 1626–1634 - PubMed
-
- Kriz W., Gretz N., Lemley K. V. (1998) Progression of glomerular diseases. Is the podocyte the culprit? Kidney Int. 54, 687–697 - PubMed
-
- Barisoni L., Kriz W., Mundel P., D'Agati V. (1999) The dysregulated podocyte phenotype. A novel concept in the pathogenesis of collapsing idiopathic focal segmental glomerulosclerosis and HIV-associated nephropathy. J. Am. Soc. Nephrol. 10, 51–61 - PubMed
-
- Barisoni L., Bruggeman L. A., Mundel P., D'Agati V. D., Klotman P. E. (2000) HIV-1 induces renal epithelial dedifferentiation in a transgenic model of HIV-associated nephropathy. Kidney Int. 58, 173–181 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01DK056492/DK/NIDDK NIH HHS/United States
- 1R01DK078897-01/DK/NIDDK NIH HHS/United States
- R01 HL084154/HL/NHLBI NIH HHS/United States
- R01HL084154/HL/NHLBI NIH HHS/United States
- T32 DK007757/DK/NIDDK NIH HHS/United States
- 5K08DK082760/DK/NIDDK NIH HHS/United States
- R01 DK078897/DK/NIDDK NIH HHS/United States
- K08 DK082760/DK/NIDDK NIH HHS/United States
- F32 DK094635/DK/NIDDK NIH HHS/United States
- P50 GM071558/GM/NIGMS NIH HHS/United States
- T32 DK07757-12/DK/NIDDK NIH HHS/United States
- P01 DK056492/DK/NIDDK NIH HHS/United States
- R01 GM098316/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

