Involvement of phosphoinositide 3-kinase and PTEN protein in mechanism of activation of TRPC6 protein in vascular smooth muscle cells
- PMID: 22493444
- PMCID: PMC3366789
- DOI: 10.1074/jbc.M112.341354
Involvement of phosphoinositide 3-kinase and PTEN protein in mechanism of activation of TRPC6 protein in vascular smooth muscle cells
Abstract
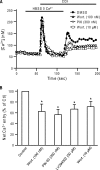
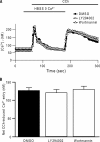
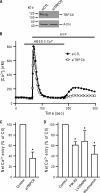
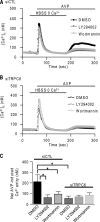
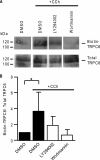
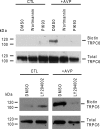
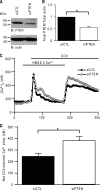
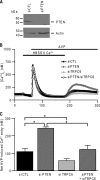
TRPC6 is a cation channel in the plasma membrane that plays a role in Ca(2+) entry after the stimulation of a G(q)-protein-coupled or tyrosine-kinase receptor. TRPC6 translocates to the plasma membrane upon stimulation and remains there as long as the stimulus is present. However, the mechanism that regulates the trafficking and activation of TRPC6 are unclear. In this study we showed phosphoinositide 3-kinase and its antagonistic phosphatase, PTEN, are involved in the activation of TRPC6. The inhibition of PI3K by PIK-93, LY294002, or wortmannin decreased carbachol-induced translocation of TRPC6 to the plasma membrane and carbachol-induced net Ca(2+) entry into T6.11 cells. Conversely, a reduction of PTEN expression did not affect carbachol-induced externalization of TRPC6 but increased Ca(2+) entry through TRPC6 in T6.11 cells. We also showed that the PI3K/PTEN pathway regulates vasopressin-induced translocation of TRPC6 to the plasma membrane and vasopressin-induced Ca(2+) entry into A7r5 cells, which endogenously express TRPC6. In summary, we provided evidence that the PI3K/PTEN pathway plays an important role in the translocation of TRPC6 to the plasma membrane and may thus have a significant impact on Ca(2+) signaling in cells that endogenously express TRPC6.
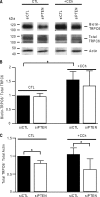
Figures
Similar articles
-
Protein kinase C-dependent phosphorylation of transient receptor potential canonical 6 (TRPC6) on serine 448 causes channel inhibition.J Biol Chem. 2010 Dec 24;285(52):40534-43. doi: 10.1074/jbc.M110.160051. Epub 2010 Oct 20. J Biol Chem. 2010. PMID: 20961851 Free PMC article.
-
cAMP activates TRPC6 channels via the phosphatidylinositol 3-kinase (PI3K)-protein kinase B (PKB)-mitogen-activated protein kinase kinase (MEK)-ERK1/2 signaling pathway.J Biol Chem. 2011 Jun 3;286(22):19439-45. doi: 10.1074/jbc.M110.210294. Epub 2011 Apr 12. J Biol Chem. 2011. PMID: 21487005 Free PMC article.
-
Calcium-sensing receptor modulates extracellular Ca(2+) entry via TRPC-encoded receptor-operated channels in human aortic smooth muscle cells.Am J Physiol Cell Physiol. 2011 Aug;301(2):C461-8. doi: 10.1152/ajpcell.00389.2010. Epub 2011 May 11. Am J Physiol Cell Physiol. 2011. PMID: 21562303 Free PMC article.
-
Phosphatidylinositol 3-kinase inhibitors: promising drug candidates for cancer therapy.Cancer Sci. 2008 Sep;99(9):1734-40. doi: 10.1111/j.1349-7006.2008.00891.x. Epub 2008 Jul 4. Cancer Sci. 2008. PMID: 18616528 Free PMC article. Review.
-
Is Zn2+ the new Ca2+ for TRPC6 channels in the myocardium?Cell Calcium. 2023 Jan;109:102674. doi: 10.1016/j.ceca.2022.102674. Epub 2022 Nov 22. Cell Calcium. 2023. PMID: 36450202 Review.
Cited by
-
PI3K Isoforms in Vascular Biology, A Focus on the Vascular System-Immune Response Connection.Curr Top Microbiol Immunol. 2022;436:289-309. doi: 10.1007/978-3-031-06566-8_12. Curr Top Microbiol Immunol. 2022. PMID: 36243849
-
RNA-seq analysis reveals TRPC genes to impact an unexpected number of metabolic and regulatory pathways.Sci Rep. 2020 Apr 29;10(1):7227. doi: 10.1038/s41598-020-61177-x. Sci Rep. 2020. PMID: 32350291 Free PMC article.
-
SNF8, a member of the ESCRT-II complex, interacts with TRPC6 and enhances its channel activity.BMC Cell Biol. 2012 Nov 21;13:33. doi: 10.1186/1471-2121-13-33. BMC Cell Biol. 2012. PMID: 23171048 Free PMC article.
-
G protein-coupled receptor signalling potentiates the osmo-mechanical activation of TRPC5 channels.Pflugers Arch. 2014 Aug;466(8):1635-46. doi: 10.1007/s00424-013-1392-z. Epub 2013 Nov 1. Pflugers Arch. 2014. PMID: 24177920
-
In pursuit of small molecule chemistry for calcium-permeable non-selective TRPC channels -- mirage or pot of gold?Br J Pharmacol. 2013 Oct;170(3):459-74. doi: 10.1111/bph.12274. Br J Pharmacol. 2013. PMID: 23763262 Free PMC article. Review.
References
-
- Berridge M. J., Bootman M. D., Lipp P. (1998) Calcium. A life and death signal. Nature 395, 645–648 - PubMed
-
- Berridge M. J., Lipp P., Bootman M. D. (2000) The versatility and universality of calcium signaling. Nat. Rev. Mol. Cell Biol. 1, 11–21 - PubMed
-
- Pedersen S. F., Owsianik G., Nilius B. (2005) TRP channels. An overview. Cell Calcium 38, 233–252 - PubMed
-
- Vazquez G., Wedel B. J., Aziz O., Trebak M., Putney J. W., Jr. (2004) The mammalian TRPC cation channels. Biochim. Biophys. Acta 1742, 21–36 - PubMed
-
- Winn M. P., Conlon P. J., Lynn K. L., Farrington M. K., Creazzo T., Hawkins A. F., Daskalakis N., Kwan S. Y., Ebersviller S., Burchette J. L., Pericak-Vance M. A., Howell D. N., Vance J. M., Rosenberg P. B. (2005) A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science 308, 1801–1804 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

