Cholesteatoma-associated fibroblasts modulate epithelial growth and differentiation through KGF/FGF7 secretion
- PMID: 22481617
- PMCID: PMC3407559
- DOI: 10.1007/s00418-012-0947-y
Cholesteatoma-associated fibroblasts modulate epithelial growth and differentiation through KGF/FGF7 secretion
Abstract
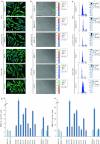
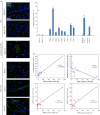
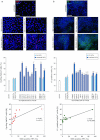
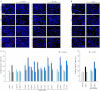
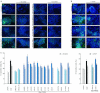
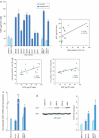
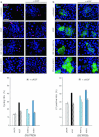
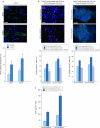
The keratinocyte growth factor (KGF/FGF7), produced by stromal cells, is a key paracrine mediator of epithelial proliferation, differentiation and migration. Expression of the growth factor is increased in wound healing and in hyperproliferative epithelial diseases, as a consequence of the activation of dermal fibroblasts by the inflammatory microenvironment. The middle ear cholesteatoma, an aural epidermal pathology characterized by keratinocyte hyperproliferation and chronic inflammation, may represent a model condition to study the epithelial-mesenchymal interactions. To develop an in vitro model for this disease, we isolated and characterized human primary cultures of fibroblasts associated with the cholesteatoma lesion, analyzing their secretory behaviour and degree of differentiation or activation. Compared to the perilesional or control normal fibroblasts, all cultures derived from cholesteatoma tissues were less proliferating and more differentiated and their highly variable activated phenotype correlated with the secretion of KGF as well as of metalloproteases 2 and 9. Culture supernatants collected from the cholesteatoma-associated fibroblasts were able to increase the proliferation and differentiation of human keratinocytes assessed by the expression of Ki67 and keratin-1 markers. The single crucial contribution of the KGF released by fibroblasts on the keratinocyte biological response was shown by the specific, although partial, block induced by inhibiting the KGF receptor or by immunoneutralizing the growth factor. Altogether, these results suggest that the activation of the stromal fibroblasts present in the pathological tissue, and the consequent increased secretion of KGF, play a crucial role in the deregulation of the epidermal proliferation and differentiation.
Figures
Similar articles
-
Keratinocyte growth factor and its receptor expression in chronic otitis media with and without cholesteatoma.Rom J Morphol Embryol. 2017;58(4):1333-1338. Rom J Morphol Embryol. 2017. PMID: 29556625
-
Keratinocyte Growth Factor (KGF) Modulates Epidermal Progenitor Cell Kinetics through Activation of p63 in Middle Ear Cholesteatoma.J Assoc Res Otolaryngol. 2018 Jun;19(3):223-241. doi: 10.1007/s10162-018-0662-z. Epub 2018 Mar 16. J Assoc Res Otolaryngol. 2018. PMID: 29549594 Free PMC article.
-
Possible involvement of keratinocyte growth factor and its receptor in enhanced epithelial-cell proliferation and acquired recurrence of middle-ear cholesteatoma.Lab Invest. 2003 Jan;83(1):123-36. doi: 10.1097/01.lab.0000050763.64145.cb. Lab Invest. 2003. PMID: 12533693
-
Keratinocyte growth factor signaling promotes stem/progenitor cell proliferation under p63 expression during middle ear cholesteatoma formation.Curr Opin Otolaryngol Head Neck Surg. 2020 Oct;28(5):291-295. doi: 10.1097/MOO.0000000000000655. Curr Opin Otolaryngol Head Neck Surg. 2020. PMID: 32796271 Review.
-
[The research progress of keratin's roles in the pathology of middle ear cholesteatoma].Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2019 Nov 7;54(11):870-874. doi: 10.3760/cma.j.issn.1673-0860.2019.11.014. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2019. PMID: 31795552 Review. Chinese.
Cited by
-
The Aberrant Expression of the Mesenchymal Variant of FGFR2 in the Epithelial Context Inhibits Autophagy.Cells. 2019 Jun 29;8(7):653. doi: 10.3390/cells8070653. Cells. 2019. PMID: 31261937 Free PMC article.
-
Upregulation of phosphorylated HSP27, PRDX2, GRP75, GRP78 and GRP94 in acquired middle ear cholesteatoma growth.Int J Mol Sci. 2013 Jul 11;14(7):14439-59. doi: 10.3390/ijms140714439. Int J Mol Sci. 2013. PMID: 23852020 Free PMC article.
-
Evaluation of the Role of Myofibroblast and Fibronectin in the Aetiopathogenesis of Cholesteatoma.Indian J Otolaryngol Head Neck Surg. 2024 Oct;76(5):4064-4073. doi: 10.1007/s12070-024-04784-8. Epub 2024 Jun 11. Indian J Otolaryngol Head Neck Surg. 2024. PMID: 39376278
-
The Histochemistry and Cell Biology compendium: a review of 2012.Histochem Cell Biol. 2013 Jun;139(6):815-46. doi: 10.1007/s00418-013-1098-5. Epub 2013 May 12. Histochem Cell Biol. 2013. PMID: 23665922 Review.
-
In vitro characterization of mitochondrial function and structure in rat and human cells with a deficiency of the NADH: ubiquinone oxidoreductase Ndufc2 subunit.Hum Mol Genet. 2017 Dec 1;26(23):4541-4555. doi: 10.1093/hmg/ddx333. Hum Mol Genet. 2017. PMID: 28973657 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

