A cost-effectiveness analysis of "test" versus "treat" patients hospitalized with suspected influenza in Hong Kong
- PMID: 22479363
- PMCID: PMC3315544
- DOI: 10.1371/journal.pone.0033123
A cost-effectiveness analysis of "test" versus "treat" patients hospitalized with suspected influenza in Hong Kong
Abstract
Background: Seasonal and 2009 H1N1 influenza viruses may cause severe diseases and result in excess hospitalization and mortality in the older and younger adults, respectively. Early antiviral treatment may improve clinical outcomes. We examined potential outcomes and costs of test-guided versus empirical treatment in patients hospitalized for suspected influenza in Hong Kong.
Methods: We designed a decision tree to simulate potential outcomes of four management strategies in adults hospitalized for severe respiratory infection suspected of influenza: "immunofluorescence-assay" (IFA) or "polymerase-chain-reaction" (PCR)-guided oseltamivir treatment, "empirical treatment plus PCR" and "empirical treatment alone". Model inputs were derived from literature. The average prevalence (11%) of influenza in 2010-2011 (58% being 2009 H1N1) among cases of respiratory infections was used in the base-case analysis. Primary outcome simulated was cost per quality-adjusted life-year (QALY) expected (ICER) from the Hong Kong healthcare providers' perspective.
Results: In base-case analysis, "empirical treatment alone" was shown to be the most cost-effective strategy and dominated the other three options. Sensitivity analyses showed that "PCR-guided treatment" would dominate "empirical treatment alone" when the daily cost of oseltamivir exceeded USD18, or when influenza prevalence was <2.5% and the predominant circulating viruses were not 2009 H1N1. Using USD50,000 as the threshold of willingness-to-pay, "empirical treatment alone" and "PCR-guided treatment" were cost-effective 97% and 3% of time, respectively, in 10,000 Monte-Carlo simulations.
Conclusions: During influenza epidemics, empirical antiviral treatment appears to be a cost-effective strategy in managing patients hospitalized with severe respiratory infection suspected of influenza, from the perspective of healthcare providers in Hong Kong.
Conflict of interest statement
Figures
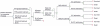


Similar articles
-
Oseltamivir Treatment for Children with Influenza-Like Illness in China: A Cost-Effectiveness Analysis.PLoS One. 2016 Apr 15;11(4):e0153664. doi: 10.1371/journal.pone.0153664. eCollection 2016. PLoS One. 2016. PMID: 27082431 Free PMC article.
-
Cost-effectiveness of molecular point-of-care testing for influenza viruses in elderly patients at ambulatory care setting.PLoS One. 2017 Jul 27;12(7):e0182091. doi: 10.1371/journal.pone.0182091. eCollection 2017. PLoS One. 2017. PMID: 28750092 Free PMC article.
-
Management of influenza in adults older than 65 years of age: cost-effectiveness of rapid testing and antiviral therapy.Ann Intern Med. 2003 Sep 2;139(5 Pt 1):321-9. doi: 10.7326/0003-4819-139-5_part_1-200309020-00007. Ann Intern Med. 2003. PMID: 12965940
-
Amantadine, oseltamivir and zanamivir for the prophylaxis of influenza (including a review of existing guidance no. 67): a systematic review and economic evaluation.Health Technol Assess. 2009 Feb;13(11):iii, ix-xii, 1-246. doi: 10.3310/hta13110. Health Technol Assess. 2009. PMID: 19215705 Review.
-
Emerging oseltamivir resistance in seasonal and pandemic influenza A/H1N1.J Clin Virol. 2011 Oct;52(2):70-8. doi: 10.1016/j.jcv.2011.05.019. Epub 2011 Jun 17. J Clin Virol. 2011. PMID: 21684202 Review.
Cited by
-
Unremarked or Unperformed? Systematic Review on Reporting of Validation Efforts of Health Economic Decision Models in Seasonal Influenza and Early Breast Cancer.Pharmacoeconomics. 2016 Sep;34(9):833-45. doi: 10.1007/s40273-016-0410-3. Pharmacoeconomics. 2016. PMID: 27129572 Free PMC article. Review.
-
Cost-Effectiveness of Antiviral Treatments for Pandemics and Outbreaks of Respiratory Illnesses, Including COVID-19: A Systematic Review of Published Economic Evaluations.Value Health. 2020 Nov;23(11):1409-1422. doi: 10.1016/j.jval.2020.07.002. Epub 2020 Sep 6. Value Health. 2020. PMID: 33127010 Free PMC article. Review.
-
The epidemiological and public health research response to 2009 pandemic influenza A(H1N1): experiences from Hong Kong.Influenza Other Respir Viruses. 2013 May;7(3):367-82. doi: 10.1111/j.1750-2659.2012.00420.x. Epub 2012 Aug 9. Influenza Other Respir Viruses. 2013. PMID: 22883352 Free PMC article. Review.
-
Utility of Rapid Influenza Molecular Testing in an Outpatient Hemodialysis Unit: A Prospective Cohort Study.Can J Kidney Health Dis. 2020 Feb 26;7:2054358120907816. doi: 10.1177/2054358120907816. eCollection 2020. Can J Kidney Health Dis. 2020. PMID: 32153798 Free PMC article.
-
Respiratory Viral Testing and Influenza Antiviral Prescriptions During Hospitalization for Acute Respiratory Illnesses.Open Forum Infect Dis. 2016 Feb 12;3(1):ofv216. doi: 10.1093/ofid/ofv216. eCollection 2016 Jan. Open Forum Infect Dis. 2016. PMID: 26885545 Free PMC article.
References
-
- Thompson WW, Shay DK, Weintraub E, Brammer L, Bridges CB, et al. Influenza-associated hospitalizations in the United States. JAMA. 2004;292:1333–1340. - PubMed
-
- Lee N, Ison MG. Diagnosis, Management and Outcomes of Adults Hospitalized with Influenza. Antiviral Therapy in press - PubMed
-
- Writing Committee of the WHO Consultation on Clinical Aspects of Pandemic (H1N1) 2009 Influenza. Clinical aspects of pandemic 2009 influenza A (H1N1) virus infection. N Engl J Med. 2010;362:1708–1719. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

