HIV-1 superinfection in women broadens and strengthens the neutralizing antibody response
- PMID: 22479183
- PMCID: PMC3315492
- DOI: 10.1371/journal.ppat.1002611
HIV-1 superinfection in women broadens and strengthens the neutralizing antibody response
Abstract
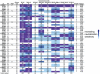
Identifying naturally-occurring neutralizing antibodies (NAb) that are cross-reactive against all global subtypes of HIV-1 is an important step toward the development of a vaccine. Establishing the host and viral determinants for eliciting such broadly NAbs is also critical for immunogen design. NAb breadth has previously been shown to be positively associated with viral diversity. Therefore, we hypothesized that superinfected individuals develop a broad NAb response as a result of increased antigenic stimulation by two distinct viruses. To test this hypothesis, plasma samples from 12 superinfected women each assigned to three singly infected women were tested against a panel of eight viruses representing four different HIV-1 subtypes at matched time points post-superinfection (~5 years post-initial infection). Here we show superinfected individuals develop significantly broader NAb responses post-superinfection when compared to singly infected individuals (RR = 1.68, CI: 1.23-2.30, p = 0.001). This was true even after controlling for NAb breadth developed prior to superinfection, contemporaneous CD4+ T cell count and viral load. Similarly, both unadjusted and adjusted analyses showed significantly greater potency in superinfected cases compared to controls. Notably, two superinfected individuals were able to neutralize variants from four different subtypes at plasma dilutions >1∶300, suggesting that their NAbs exhibit elite activity. Cross-subtype breadth was detected within a year of superinfection in both of these individuals, which was within 1.5 years of their initial infection. These data suggest that sequential infections lead to augmentation of the NAb response, a process that may provide insight into potential mechanisms that contribute to the development of antibody breadth. Therefore, a successful vaccination strategy that mimics superinfection may lead to the development of broad NAbs in immunized individuals.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



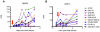
Similar articles
-
The Broad Neutralizing Antibody Responses after HIV-1 Superinfection Are Not Dominated by Antibodies Directed to Epitopes Common in Single Infection.PLoS Pathog. 2015 Jul 9;11(7):e1004973. doi: 10.1371/journal.ppat.1004973. eCollection 2015 Jul. PLoS Pathog. 2015. PMID: 26158467 Free PMC article.
-
Intrasubtype B HIV-1 Superinfection Correlates with Delayed Neutralizing Antibody Response.J Virol. 2017 Aug 10;91(17):e00475-17. doi: 10.1128/JVI.00475-17. Print 2017 Sep 1. J Virol. 2017. PMID: 28615205 Free PMC article.
-
HIV-1 subtype C superinfected individuals mount low autologous neutralizing antibody responses prior to intrasubtype superinfection.Retrovirology. 2012 Sep 20;9:76. doi: 10.1186/1742-4690-9-76. Retrovirology. 2012. PMID: 22995123 Free PMC article.
-
Transmission of HIV-1 in the face of neutralizing antibodies.Curr HIV Res. 2007 Nov;5(6):578-87. doi: 10.2174/157016207782418461. Curr HIV Res. 2007. PMID: 18045114 Review.
-
Role of human immunodeficiency virus type 1 envelope structure in the induction of broadly neutralizing antibodies.J Virol. 2012 Dec;86(24):13152-63. doi: 10.1128/JVI.01110-12. Epub 2012 Sep 26. J Virol. 2012. PMID: 23015715 Free PMC article. Review.
Cited by
-
HIV-1 infections with multiple founders associate with the development of neutralization breadth.PLoS Pathog. 2022 Mar 18;18(3):e1010369. doi: 10.1371/journal.ppat.1010369. eCollection 2022 Mar. PLoS Pathog. 2022. PMID: 35303045 Free PMC article.
-
Virological and Immunological Outcomes of Coinfections.Clin Microbiol Rev. 2018 Jul 5;31(4):e00111-17. doi: 10.1128/CMR.00111-17. Print 2018 Oct. Clin Microbiol Rev. 2018. PMID: 29976554 Free PMC article. Review.
-
Early development of broadly neutralizing antibodies in HIV-1-infected infants.Nat Med. 2014 Jun;20(6):655-8. doi: 10.1038/nm.3565. Epub 2014 May 25. Nat Med. 2014. PMID: 24859529 Free PMC article.
-
Functional development of a V3/glycan-specific broadly neutralizing antibody isolated from a case of HIV superinfection.Elife. 2021 Jul 15;10:e68110. doi: 10.7554/eLife.68110. Elife. 2021. PMID: 34263727 Free PMC article.
-
Structural insights on the role of antibodies in HIV-1 vaccine and therapy.Cell. 2014 Feb 13;156(4):633-48. doi: 10.1016/j.cell.2014.01.052. Cell. 2014. PMID: 24529371 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

