POF regulates the expression of genes on the fourth chromosome in Drosophila melanogaster by binding to nascent RNA
- PMID: 22473994
- PMCID: PMC3372238
- DOI: 10.1128/MCB.06622-11
POF regulates the expression of genes on the fourth chromosome in Drosophila melanogaster by binding to nascent RNA
Abstract
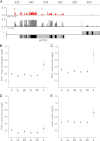
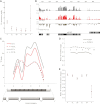
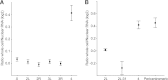
In Drosophila, two chromosome-wide compensatory systems have been characterized: the dosage compensation system that acts on the male X chromosome and the chromosome-specific regulation of genes located on the heterochromatic fourth chromosome. Dosage compensation in Drosophila is accomplished by hypertranscription of the single male X chromosome mediated by the male-specific lethal (MSL) complex. The mechanism of this compensation is suggested to involve enhanced transcriptional elongation mediated by the MSL complex, while the mechanism of compensation mediated by the painting of fourth (POF) protein on the fourth chromosome has remained elusive. Here, we show that POF binds to nascent RNA, and this binding is associated with increased transcription output from chromosome 4. We also show that genes located in heterochromatic regions spend less time in transition from the site of transcription to the nuclear envelope. These results provide useful insights into the means by which genes in heterochromatic regions can overcome the repressive influence of their hostile environment.
Figures


Similar articles
-
Targeting of Painting of fourth to roX1 and roX2 proximal sites suggests evolutionary links between dosage compensation and the regulation of the fourth chromosome in Drosophila melanogaster.G3 (Bethesda). 2013 Aug 7;3(8):1325-34. doi: 10.1534/g3.113.006866. G3 (Bethesda). 2013. PMID: 23733888 Free PMC article.
-
Painting of fourth and chromosome-wide regulation of the 4th chromosome in Drosophila melanogaster.EMBO J. 2007 May 2;26(9):2307-16. doi: 10.1038/sj.emboj.7601604. Epub 2007 Feb 22. EMBO J. 2007. PMID: 17318176 Free PMC article.
-
Painting of Fourth and the X-Linked 1.688 Satellite in D. melanogaster is Involved in Chromosome-Wide Gene Regulation.Cells. 2020 Jan 30;9(2):323. doi: 10.3390/cells9020323. Cells. 2020. PMID: 32019091 Free PMC article.
-
The right dose for every sex.Chromosoma. 2007 Apr;116(2):95-106. doi: 10.1007/s00412-006-0089-x. Epub 2006 Nov 24. Chromosoma. 2007. PMID: 17124606 Free PMC article. Review.
-
Chromatin mechanisms in Drosophila dosage compensation.Prog Mol Subcell Biol. 2005;38:123-49. doi: 10.1007/3-540-27310-7_5. Prog Mol Subcell Biol. 2005. PMID: 15881893 Review.
Cited by
-
Global chromatin conformation differences in the Drosophila dosage compensated chromosome X.Nat Commun. 2019 Nov 25;10(1):5355. doi: 10.1038/s41467-019-13350-8. Nat Commun. 2019. PMID: 31767860 Free PMC article.
-
The variant histone H2A.V of Drosophila--three roles, two guises.Chromosoma. 2013 Aug;122(4):245-58. doi: 10.1007/s00412-013-0409-x. Epub 2013 Apr 4. Chromosoma. 2013. PMID: 23553272 Review.
-
Proximity ligation assays of protein and RNA interactions in the male-specific lethal complex on Drosophila melanogaster polytene chromosomes.Chromosoma. 2015 Sep;124(3):385-95. doi: 10.1007/s00412-015-0509-x. Epub 2015 Feb 19. Chromosoma. 2015. PMID: 25694028 Free PMC article.
-
Enrichment of HP1a on Drosophila chromosome 4 genes creates an alternate chromatin structure critical for regulation in this heterochromatic domain.PLoS Genet. 2012 Sep;8(9):e1002954. doi: 10.1371/journal.pgen.1002954. Epub 2012 Sep 20. PLoS Genet. 2012. PMID: 23028361 Free PMC article.
-
HP1a recruitment to promoters is independent of H3K9 methylation in Drosophila melanogaster.PLoS Genet. 2012;8(11):e1003061. doi: 10.1371/journal.pgen.1003061. Epub 2012 Nov 15. PLoS Genet. 2012. PMID: 23166515 Free PMC article.
References
-
- Akhtar A, Zink D, Becker PB. 2000. Chromodomains are protein-RNA interaction modules. Nature 407:405–409 - PubMed
-
- Ashburner M, Golic KG, Hawley RS. 2005. Drosophila: a laboratory handbook. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
