Aquaporin-4 deficiency impairs synaptic plasticity and associative fear memory in the lateral amygdala: involvement of downregulation of glutamate transporter-1 expression
- PMID: 22473056
- PMCID: PMC3376319
- DOI: 10.1038/npp.2012.34
Aquaporin-4 deficiency impairs synaptic plasticity and associative fear memory in the lateral amygdala: involvement of downregulation of glutamate transporter-1 expression
Abstract
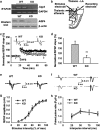
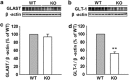
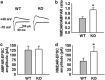
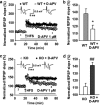
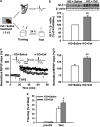
Astrocytes are implicated in information processing, signal transmission, and regulation of synaptic plasticity. Aquaporin-4 (AQP4) is the major water channel in adult brain and is primarily expressed in astrocytes. A growing body of evidence indicates that AQP4 is a potential molecular target for the regulation of astrocytic function. However, little is known about the role of AQP4 in synaptic plasticity in the amygdala. Therefore, we evaluated long-term potentiation (LTP) in the lateral amygdala (LA) and associative fear memory of AQP4 knockout (KO) and wild-type mice. We found that AQP4 deficiency impaired LTP in the thalamo-LA pathway and associative fear memory. Furthermore, AQP4 deficiency significantly downregulated glutamate transporter-1 (GLT-1) expression and selectively increased NMDA receptor (NMDAR)-mediated EPSCs in the LA. However, low concentration of NMDAR antagonist reversed the impairment of LTP in KO mice. Upregulating GLT-1 expression by chronic treatment with ceftriaxone also reversed the impairment of LTP and fear memory in KO mice. These findings imply a role for AQP4 in synaptic plasticity and associative fear memory in the amygdala by regulating GLT-1 expression.
Figures

Similar articles
-
Chronic ceftriaxone treatment rescues hippocampal memory deficit in AQP4 knockout mice via activation of GLT-1.Neuropharmacology. 2013 Dec;75:213-22. doi: 10.1016/j.neuropharm.2013.08.009. Epub 2013 Aug 22. Neuropharmacology. 2013. PMID: 23973312
-
Postsynaptic BDNF signalling regulates long-term potentiation at thalamo-amygdala afferents.J Physiol. 2012 Jan 1;590(1):193-208. doi: 10.1113/jphysiol.2011.220434. Epub 2011 Nov 14. J Physiol. 2012. PMID: 22083603 Free PMC article.
-
Spatiotemporal asymmetry of associative synaptic plasticity in fear conditioning pathways.Neuron. 2006 Dec 7;52(5):883-96. doi: 10.1016/j.neuron.2006.10.010. Neuron. 2006. PMID: 17145508 Free PMC article.
-
Synaptic mechanisms of associative memory in the amygdala.Neuron. 2005 Sep 15;47(6):783-6. doi: 10.1016/j.neuron.2005.08.009. Neuron. 2005. PMID: 16157273 Review.
-
Aquaporin-4 water channels and synaptic plasticity in the hippocampus.Neurochem Int. 2013 Dec;63(7):702-11. doi: 10.1016/j.neuint.2013.05.003. Epub 2013 May 15. Neurochem Int. 2013. PMID: 23684954 Free PMC article. Review.
Cited by
-
From pathophysiology to novel antidepressant drugs: glial contributions to the pathology and treatment of mood disorders.Biol Psychiatry. 2013 Jun 15;73(12):1172-9. doi: 10.1016/j.biopsych.2013.03.032. Biol Psychiatry. 2013. PMID: 23726152 Free PMC article. Review.
-
Restoring visual function to blind mice with a photoswitch that exploits electrophysiological remodeling of retinal ganglion cells.Neuron. 2014 Feb 19;81(4):800-13. doi: 10.1016/j.neuron.2014.01.003. Neuron. 2014. PMID: 24559673 Free PMC article.
-
The Role of Astrocytic Aquaporin-4 in Synaptic Plasticity and Learning and Memory.Front Integr Neurosci. 2016 Feb 24;10:8. doi: 10.3389/fnint.2016.00008. eCollection 2016. Front Integr Neurosci. 2016. PMID: 26941623 Free PMC article. Review.
-
Fasudil attenuates lipopolysaccharide-induced cognitive impairment in C57BL/6 mice through anti-oxidative and anti-inflammatory effects: Possible role of aquaporin-4.IBRO Neurosci Rep. 2024 Oct 24;17:372-381. doi: 10.1016/j.ibneur.2024.10.004. eCollection 2024 Dec. IBRO Neurosci Rep. 2024. PMID: 39534317 Free PMC article.
-
Neuropilin-1 Mediates SARS-CoV-2 Infection of Astrocytes in Brain Organoids, Inducing Inflammation Leading to Dysfunction and Death of Neurons.mBio. 2022 Dec 20;13(6):e0230822. doi: 10.1128/mbio.02308-22. Epub 2022 Oct 31. mBio. 2022. PMID: 36314791 Free PMC article.
References
-
- Abel T, Lattal KM. Molecular mechanisms of memory acquisition, consolidation and retrieval. Curr Opin Neurobiol. 2001;11:180–187. - PubMed
-
- Amara SG, Fontana AC. Excitatory amino acid transporters: keeping up with glutamate. Neurochem Int. 2002;41:313–318. - PubMed
-
- Amiry-Moghaddam M, Ottersen OP. The molecular basis of water transport in the brain. Nat Rev Neurosci. 2003;4:991–1001. - PubMed
-
- Bao F, Chen M, Zhang Y, Zhao Z. Hypoalgesia in mice lacking aquaporin-4 water channels. Brain Res Bull. 2010;83:298–303. - PubMed
-
- Barad M, Gean PW, Lutz B. The role of the amygdala in the extinction of conditioned fear. Biol Psychiatry. 2006;60:322–328. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

