cPLA2α and EHD1 interact and regulate the vesiculation of cholesterol-rich, GPI-anchored, protein-containing endosomes
- PMID: 22456504
- PMCID: PMC3350552
- DOI: 10.1091/mbc.E11-10-0881
cPLA2α and EHD1 interact and regulate the vesiculation of cholesterol-rich, GPI-anchored, protein-containing endosomes
Abstract
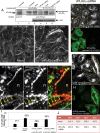
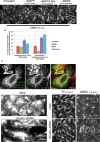
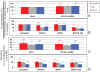
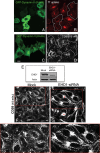
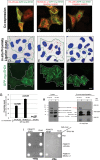
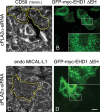
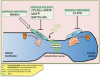
The lipid modifier phospholipase A2 catalyzes the hydrolysis of phospholipids to inverted-cone-shaped lysophospholipids that contribute to membrane curvature and/or tubulation. Conflicting findings exist regarding the function of cytosolic phospholipase A2 (cPLA2) and its role in membrane regulation at the Golgi and early endosomes. However, no studies addressed the role of cPLA2 in the regulation of cholesterol-rich membranes that contain glycosylphosphatidylinositol-anchored proteins (GPI-APs). Our studies support a role for cPLA2α in the vesiculation of GPI-AP-containing membranes, using endogenous CD59 as a model for GPI-APs. On cPLA2α depletion, CD59-containing endosomes became hypertubular. Moreover, accumulation of lysophospholipids induced by a lysophospholipid acyltransferase inhibitor extensively vesiculated CD59-containing endosomes. However, overexpression of cPLA2α did not increase the endosomal vesiculation, implying a requirement for additional factors. Indeed, depletion of the "pinchase" EHD1, a C-terminal Eps15 homology domain (EHD) ATPase, also induced hypertubulation of CD59-containing endosomes. Furthermore, EHD1 and cPLA2α demonstrated in situ proximity (<40 nm) and interacted in vivo. The results presented here provide evidence that the lipid modifier cPLA2α and EHD1 are involved in the vesiculation of CD59-containing endosomes. We speculate that cPLA2α induces membrane curvature and allows EHD1, possibly in the context of a complex, to sever the curved membranes into vesicles.
Figures

Similar articles
-
Differential roles of C-terminal Eps15 homology domain proteins as vesiculators and tubulators of recycling endosomes.J Biol Chem. 2013 Oct 18;288(42):30172-30180. doi: 10.1074/jbc.M113.488627. Epub 2013 Sep 9. J Biol Chem. 2013. PMID: 24019528 Free PMC article.
-
Novel functions for the endocytic regulatory proteins MICAL-L1 and EHD1 in mitosis.Traffic. 2015 Jan;16(1):48-67. doi: 10.1111/tra.12234. Epub 2014 Nov 14. Traffic. 2015. PMID: 25287187 Free PMC article.
-
Pre-sorting endosomal transport of the GPI-anchored protein, CD59, is regulated by EHD1.Traffic. 2011 Jan;12(1):102-20. doi: 10.1111/j.1600-0854.2010.01135.x. Epub 2010 Nov 12. Traffic. 2011. PMID: 20961375
-
Cytosolic phospholipase A2 and lysophospholipid acyltransferases.Biochim Biophys Acta Mol Cell Biol Lipids. 2019 Jun;1864(6):838-845. doi: 10.1016/j.bbalip.2018.08.006. Epub 2018 Aug 9. Biochim Biophys Acta Mol Cell Biol Lipids. 2019. PMID: 30905348 Review.
-
The Lipids of the Early Endosomes: Making Multimodality Work.Chembiochem. 2017 Jun 19;18(12):1053-1060. doi: 10.1002/cbic.201700046. Epub 2017 May 10. Chembiochem. 2017. PMID: 28374483 Review.
Cited by
-
Advances and challenges in understanding endosomal sorting and fission.FEBS J. 2023 Sep;290(17):4187-4195. doi: 10.1111/febs.16687. Epub 2022 Dec 7. FEBS J. 2023. PMID: 36413090 Free PMC article.
-
ATP-dependent membrane remodeling links EHD1 functions to endocytic recycling.Nat Commun. 2018 Dec 5;9(1):5187. doi: 10.1038/s41467-018-07586-z. Nat Commun. 2018. PMID: 30518883 Free PMC article.
-
Coronin2A links actin-based endosomal processes to the EHD1 fission machinery.Mol Biol Cell. 2022 Oct 1;33(12):ar107. doi: 10.1091/mbc.E21-12-0624. Epub 2022 Aug 3. Mol Biol Cell. 2022. PMID: 35921168 Free PMC article.
-
The enigmatic endosome - sorting the ins and outs of endocytic trafficking.J Cell Sci. 2018 Jul 6;131(13):jcs216499. doi: 10.1242/jcs.216499. J Cell Sci. 2018. PMID: 29980602 Free PMC article. Review.
-
C2 domain membrane penetration by group IVA cytosolic phospholipase A₂ induces membrane curvature changes.J Lipid Res. 2012 Dec;53(12):2656-66. doi: 10.1194/jlr.M030718. Epub 2012 Sep 18. J Lipid Res. 2012. PMID: 22991194 Free PMC article.
References
-
- Bate C, Ingham V, Williams A. Inhibition of phospholipase A(2) increased the removal of the prion derived peptide PrP82-146 from cultured neurons. Neuropharmacology. 2011;60:365–372. - PubMed
-
- Brown WJ, Chambers K, Doody A. Phospholipase A2 (PLA2) enzymes in membrane trafficking: mediators of membrane shape and function. Traffic. 2003;4:214–221. - PubMed
-
- Cai B, Katafiasz D, Horejsi V, Naslavsky N. Pre-sorting endosomal transport of the GPI-anchored protein, CD59, is regulated by EHD1. Traffic. 2011;12:102–120. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

