Nuclear IRS-1 and cancer
- PMID: 22454254
- PMCID: PMC3615708
- DOI: 10.1002/jcp.24019
Nuclear IRS-1 and cancer
Abstract
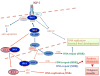
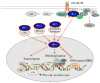
The family of insulin receptor substrates (IRS) consists of four proteins (IRS-1-IRS-4), which were initially characterized as typical cytosolic adaptor proteins involved in insulin receptor (IR) and insulin-like growth factor I receptor (IGF-IR) signaling. The first cloned and characterized member of the IRS family, IRS-1, has a predicted molecular weight of 132 kDa, however, as a result of its extensive serine phosphorylation it separates on a SDS gel as a band of approximately 160-185 kDa. In addition to its metabolic and growth-promoting functions, IRS-1 is also suspected to play a role in malignant transformation. The mechanism by which IRS-1 supports tumor growth is not fully understood, and the argument that IRS-1 merely amplifies the signal from the IGF-1R and/or IR requires further investigation. Almost a decade ago, we reported the presence of nuclear IRS-1 in medulloblastoma clinical samples, which express viral oncoprotein, large T-antigen of human polyomavirus JC (JCV T-antigen). This first demonstration of nuclear IRS-1 was confirmed by several other laboratories. Nuclear IRS-1 was also detected by cells expressing the SV40 T-antigen, v-Src, in immortalized fibroblasts stimulated with IGF-I, in hepatocytes, 32D cells, and in an osteosarcoma cell line. More recently, nuclear IRS-1 was detected in breast cancer cells in association with estrogen receptor alpha (ERα), and in JC virus negative medulloblastoma cells expressing estrogen receptor beta (ERβ), further implicating nuclear IRS-1 in cellular transformation. Here, we discuss how nuclear IRS-1 acting on DNA repair fidelity, transcriptional activity, and cell growth can support tumor development and progression.
Copyright © 2011 Wiley Periodicals, Inc.
Figures



Similar articles
-
IRS-1-Rad51 nuclear interaction sensitizes JCV T-antigen positive medulloblastoma cells to genotoxic treatment.Int J Cancer. 2006 Aug 1;119(3):539-48. doi: 10.1002/ijc.21828. Int J Cancer. 2006. PMID: 16572421
-
Estrogen receptor beta-mediated nuclear interaction between IRS-1 and Rad51 inhibits homologous recombination directed DNA repair in medulloblastoma.J Cell Physiol. 2009 May;219(2):392-401. doi: 10.1002/jcp.21683. J Cell Physiol. 2009. PMID: 19117011 Free PMC article.
-
Transformation by the simian virus 40 T antigen is regulated by IGF-I receptor and IRS-1 signaling.Oncogene. 2006 Jan 5;25(1):32-42. doi: 10.1038/sj.onc.1209013. Oncogene. 2006. PMID: 16170362
-
T-antigen of human polyomavirus JC cooperates withIGF-IR signaling system in cerebellar tumors of the childhood-medulloblastomas.Anticancer Res. 2003 May-Jun;23(3A):2035-41. Anticancer Res. 2003. PMID: 12894576 Review.
-
Insulin-like growth factor I receptor signaling system in JC virus T antigen-induced primitive neuroectodermal tumors--medulloblastomas.J Neurovirol. 2002 Dec;8 Suppl 2:138-47. doi: 10.1080/13550280290101111. J Neurovirol. 2002. PMID: 12491166 Review.
Cited by
-
Human polyomavirus modulation of the host DNA damage response.Virus Genes. 2020 Apr;56(2):128-135. doi: 10.1007/s11262-020-01736-6. Epub 2020 Jan 29. Virus Genes. 2020. PMID: 31997082 Review.
-
Analyses of Genes Critical to Tumor Survival Reveal Potential 'Supertargets': Focus on Transcription.Cancers (Basel). 2023 Jun 3;15(11):3042. doi: 10.3390/cancers15113042. Cancers (Basel). 2023. PMID: 37297004 Free PMC article.
-
Polycyclic aromatic hydrocarbons-induced ROS accumulation enhances mutagenic potential of T-antigen from human polyomavirus JC.J Cell Physiol. 2013 Nov;228(11):2127-38. doi: 10.1002/jcp.24375. J Cell Physiol. 2013. PMID: 23558788 Free PMC article.
-
Glucocorticoid receptor-IRS-1 axis controls EMT and the metastasis of breast cancers.J Mol Cell Biol. 2019 Dec 19;11(12):1042-1055. doi: 10.1093/jmcb/mjz001. J Mol Cell Biol. 2019. PMID: 30726932 Free PMC article.
-
Insulin and IGF1 signalling pathways in human astrocytes in vitro and in vivo; characterisation, subcellular localisation and modulation of the receptors.Mol Brain. 2015 Aug 22;8:51. doi: 10.1186/s13041-015-0138-6. Mol Brain. 2015. PMID: 26297026 Free PMC article.
References
-
- Araki E, Lipes MA, Patti ME, Bruning JC, Haag B, 3rd, Johnson RS, Kahn CR. Alternative pathway of insulin signalling in mice with targeted disruption of the IRS-1 gene. Nature. 1994;372(6502):186–190. - PubMed
-
- Baker J, Liu JP, Robertson EJ, Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993;75(1):73–82. - PubMed
-
- Beitner-Johnson D, Blakesley VA, Shen-Orr Z, Jimenez M, Stannard B, Wang LM, Pierce J, LeRoith D. The proto-oncogene product c-Crk associates with insulin receptor substrate-1 and 4PS. Modulation by insulin growth factor-I (IGF) and enhanced IGF-I signaling. J Biol Chem. 1996;271(16):9287–9290. - PubMed
-
- Bjornholm M, He AR, Attersand A, Lake S, Liu SC, Lienhard GE, Taylor S, Arner P, Zierath JR. Absence of functional insulin receptor substrate-3 (IRS-3) gene in humans. Diabetologia. 2002;45(12):1697–1702. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

