Drosophila Mgr, a Prefoldin subunit cooperating with von Hippel Lindau to regulate tubulin stability
- PMID: 22451918
- PMCID: PMC3326472
- DOI: 10.1073/pnas.1108537109
Drosophila Mgr, a Prefoldin subunit cooperating with von Hippel Lindau to regulate tubulin stability
Erratum in
-
Correction for Delgehyr et al., Drosophila Mgr, a Prefoldin subunit cooperating with von Hippel Lindau to regulate tubulin stability.Proc Natl Acad Sci U S A. 2016 Nov 29;113(48):E7867-E7868. doi: 10.1073/pnas.1617441113. Epub 2016 Nov 14. Proc Natl Acad Sci U S A. 2016. PMID: 27849574 Free PMC article. No abstract available.
Abstract
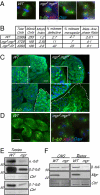
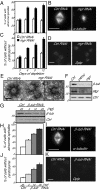
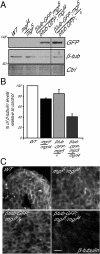
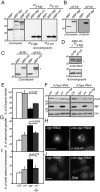
Mutations in Drosophila merry-go-round (mgr) have been known for over two decades to lead to circular mitotic figures and loss of meiotic spindle integrity. However, the identity of its gene product has remained undiscovered. We now show that mgr encodes the Prefoldin subunit counterpart of human von Hippel Lindau binding-protein 1. Depletion of Mgr from cultured cells also leads to formation of monopolar and abnormal spindles and centrosome loss. These phenotypes are associated with reductions of tubulin levels in both mgr flies and mgr RNAi-treated cultured cells. Moreover, mgr spindle defects can be phenocopied by depleting β-tubulin, suggesting Mgr function is required for tubulin stability. Instability of β-tubulin in the mgr larval brain is less pronounced than in either mgr testes or in cultured cells. However, expression of transgenic β-tubulin in the larval brain leads to increased tubulin instability, indicating that Prefoldin might only be required when tubulins are synthesized at high levels. Mgr interacts with Drosophila von Hippel Lindau protein (Vhl). Both proteins interact with unpolymerized tubulins, suggesting they cooperate in regulating tubulin functions. Accordingly, codepletion of Vhl with Mgr gives partial rescue of tubulin instability, monopolar spindle formation, and loss of centrosomes, leading us to propose a requirement for Vhl to promote degradation of incorrectly folded tubulin in the absence of functional Prefoldin. Thus, Vhl may play a pivotal role: promoting microtubule stabilization when tubulins are correctly folded by Prefoldin and tubulin destruction when they are not.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Misato Controls Mitotic Microtubule Generation by Stabilizing the TCP-1 Tubulin Chaperone Complex [corrected].Curr Biol. 2015 Jun 29;25(13):1777-83. doi: 10.1016/j.cub.2015.05.033. Epub 2015 Jun 18. Curr Biol. 2015. PMID: 26096973 Free PMC article.
-
The Drosophila gamma-tubulin small complex subunit Dgrip84 is required for structural and functional integrity of the spindle apparatus.Mol Biol Cell. 2006 Jan;17(1):272-82. doi: 10.1091/mbc.e05-08-0722. Epub 2005 Oct 19. Mol Biol Cell. 2006. PMID: 16236791 Free PMC article.
-
Augmin: a protein complex required for centrosome-independent microtubule generation within the spindle.J Cell Biol. 2008 May 5;181(3):421-9. doi: 10.1083/jcb.200711053. Epub 2008 Apr 28. J Cell Biol. 2008. PMID: 18443220 Free PMC article.
-
Gamma-tubulin at ten: progress and prospects.Cell Struct Funct. 1999 Oct;24(5):365-72. doi: 10.1247/csf.24.365. Cell Struct Funct. 1999. PMID: 15216894 Review.
-
The relative roles of centrosomal and kinetochore-driven microtubules in Drosophila spindle formation.Exp Cell Res. 2012 Jul 15;318(12):1375-80. doi: 10.1016/j.yexcr.2012.05.001. Epub 2012 May 8. Exp Cell Res. 2012. PMID: 22580224 Review.
Cited by
-
O-fucose monosaccharide of Drosophila Notch has a temperature-sensitive function and cooperates with O-glucose glycan in Notch transport and Notch signaling activation.J Biol Chem. 2015 Jan 2;290(1):505-19. doi: 10.1074/jbc.M114.616847. Epub 2014 Nov 5. J Biol Chem. 2015. PMID: 25378397 Free PMC article.
-
Prefoldin and Pins synergistically regulate asymmetric division and suppress dedifferentiation.Sci Rep. 2016 Mar 30;6:23735. doi: 10.1038/srep23735. Sci Rep. 2016. PMID: 27025979 Free PMC article.
-
The Role of Prefoldin and Its Subunits in Tumors and Their Application Prospects in Nanomedicine.Cancer Manag Res. 2020 Sep 23;12:8847-8856. doi: 10.2147/CMAR.S270237. eCollection 2020. Cancer Manag Res. 2020. PMID: 33061580 Free PMC article. Review.
-
Prefoldin Subunits and Its Associate Partners: Conservations and Specificities in Plants.Plants (Basel). 2024 Feb 18;13(4):556. doi: 10.3390/plants13040556. Plants (Basel). 2024. PMID: 38498526 Free PMC article. Review.
-
The prefoldin complex stabilizes the von Hippel-Lindau protein against aggregation and degradation.PLoS Genet. 2020 Nov 2;16(11):e1009183. doi: 10.1371/journal.pgen.1009183. eCollection 2020 Nov. PLoS Genet. 2020. PMID: 33137104 Free PMC article.
References
-
- Lundin VF, Leroux MR, Stirling PC. Quality control of cytoskeletal proteins and human disease. Trends Biochem Sci. 2010;35:288–297. - PubMed
-
- Siegert R, Leroux MR, Scheufler C, Hartl FU, Moarefi I. Structure of the molecular chaperone prefoldin: Unique interaction of multiple coiled coil tentacles with unfolded proteins. Cell. 2000;103:621–632. - PubMed
-
- Vainberg IE, et al. Prefoldin, a chaperone that delivers unfolded proteins to cytosolic chaperonin. Cell. 1998;93:863–873. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

