Naive T cells are dispensable for memory CD4+ T cell homeostasis in progressive simian immunodeficiency virus infection
- PMID: 22451717
- PMCID: PMC3328373
- DOI: 10.1084/jem.20112071
Naive T cells are dispensable for memory CD4+ T cell homeostasis in progressive simian immunodeficiency virus infection
Abstract
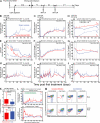
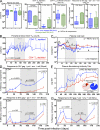
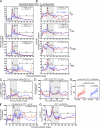
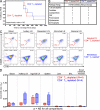
The development of AIDS in chronic HIV/simian immunodeficiency virus (SIV) infection has been closely linked to progressive failure of CD4(+) memory T cell (T(M)) homeostasis. CD4(+) naive T cells (T(N)) also decline in these infections, but their contribution to disease progression is less clear. We assessed the role of CD4(+) T(N) in SIV pathogenesis using rhesus macaques (RMs) selectively and permanently depleted of CD4(+) T(N) before SIV infection. CD4(+) T(N)-depleted and CD4(+) T(N)-repleted RMs were created by subjecting juvenile RMs to thymectomy versus sham surgery, respectively, followed by total CD4(+) T cell depletion and recovery from this depletion. Although thymectomized and sham-treated RMs manifested comparable CD4(+) T(M) recovery, only sham-treated RMs reconstituted CD4(+) T(N). CD4(+) T(N)-depleted RMs responded to SIVmac239 infection with markedly attenuated SIV-specific CD4(+) T cell responses, delayed SIVenv-specific Ab responses, and reduced SIV-specific CD8(+) T cell responses. However, CD4(+) T(N)-depleted and -repleted groups showed similar levels of SIV replication. Moreover, CD4(+) T(N) deficiency had no significant effect on CD4(+) T(M) homeostasis (either on or off anti-retroviral therapy) or disease progression. These data demonstrate that the CD4(+) T(N) compartment is dispensable for CD4(+) T(M) homeostasis in progressive SIV infection, and they confirm that CD4(+) T(M) comprise a homeostatically independent compartment that is intrinsically capable of self-renewal.
Figures
Similar articles
-
Insufficient production and tissue delivery of CD4+ memory T cells in rapidly progressive simian immunodeficiency virus infection.J Exp Med. 2004 Nov 15;200(10):1299-314. doi: 10.1084/jem.20041049. J Exp Med. 2004. PMID: 15545355 Free PMC article.
-
Increased stability and limited proliferation of CD4+ central memory T cells differentiate nonprogressive simian immunodeficiency virus (SIV) infection of sooty mangabeys from progressive SIV infection of rhesus macaques.J Virol. 2014 Apr;88(8):4533-42. doi: 10.1128/JVI.03515-13. Epub 2014 Feb 5. J Virol. 2014. PMID: 24501416 Free PMC article.
-
Initiation of Antiretroviral Therapy Restores CD4+ T Memory Stem Cell Homeostasis in Simian Immunodeficiency Virus-Infected Macaques.J Virol. 2016 Jul 11;90(15):6699-6708. doi: 10.1128/JVI.00492-16. Print 2016 Aug 1. J Virol. 2016. PMID: 27170752 Free PMC article.
-
Antiviral CD8+ T cells in the genital tract control viral replication and delay progression to AIDS after vaginal SIV challenge in rhesus macaques immunized with virulence attenuated SHIV 89.6.J Intern Med. 2009 Jan;265(1):67-77. doi: 10.1111/j.1365-2796.2008.02051.x. J Intern Med. 2009. PMID: 19093961 Free PMC article. Review.
-
Limited role for the thymus in SIV pathogenesis.Eur J Immunol. 2005 Jan;35(1):42-5. doi: 10.1002/eji.200425643. Eur J Immunol. 2005. PMID: 15593304 Review.
Cited by
-
Simian-Human Immunodeficiency Virus SHIV.CH505 Infection of Rhesus Macaques Results in Persistent Viral Replication and Induces Intestinal Immunopathology.J Virol. 2019 Aug 28;93(18):e00372-19. doi: 10.1128/JVI.00372-19. Print 2019 Sep 15. J Virol. 2019. PMID: 31217249 Free PMC article.
-
Host-pathogen interaction in HIV infection.Curr Opin Immunol. 2013 Aug;25(4):463-9. doi: 10.1016/j.coi.2013.07.003. Epub 2013 Jul 24. Curr Opin Immunol. 2013. PMID: 23890585 Free PMC article. Review.
-
Impact of alemtuzumab-mediated lymphocyte depletion on SIV reservoir establishment and persistence.PLoS Pathog. 2024 Aug 22;20(8):e1012496. doi: 10.1371/journal.ppat.1012496. eCollection 2024 Aug. PLoS Pathog. 2024. PMID: 39173097 Free PMC article.
-
Effect of IL-7 Therapy on Naive and Memory T Cell Homeostasis in Aged Rhesus Macaques.J Immunol. 2015 Nov 1;195(9):4292-305. doi: 10.4049/jimmunol.1500609. Epub 2015 Sep 28. J Immunol. 2015. PMID: 26416281 Free PMC article.
-
Cytomegalovirus (CMV) Epitope-Specific CD4+ T Cells Are Inflated in HIV+ CMV+ Subjects.J Immunol. 2017 Nov 1;199(9):3187-3201. doi: 10.4049/jimmunol.1700851. Epub 2017 Oct 2. J Immunol. 2017. PMID: 28972094 Free PMC article.
References
-
- Arron S.T., Ribeiro R.M., Gettie A., Bohm R., Blanchard J., Yu J., Perelson A.S., Ho D.D., Zhang L. 2005. Impact of thymectomy on the peripheral T cell pool in rhesus macaques before and after infection with simian immunodeficiency virus. Eur. J. Immunol. 35:46–55 10.1002/eji.200424996 - DOI - PubMed
-
- Brenchley J.M., Schacker T.W., Ruff L.E., Price D.A., Taylor J.H., Beilman G.J., Nguyen P.L., Khoruts A., Larson M., Haase A.T., Douek D.C. 2004. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J. Exp. Med. 200:749–759 10.1084/jem.20040874 - DOI - PMC - PubMed
-
- Catalfamo M., Wilhelm C., Tcheung L., Proschan M., Friesen T., Park J.H., Adelsberger J., Baseler M., Maldarelli F., Davey R., et al. 2011. CD4 and CD8 T cell immune activation during chronic HIV infection: roles of homeostasis, HIV, type I IFN, and IL-7. J. Immunol. 186:2106–2116 10.4049/jimmunol.1002000 - DOI - PMC - PubMed
-
- Cline A.N., Bess J.W., Piatak M., Jr, Lifson J.D. 2005. Highly sensitive SIV plasma viral load assay: practical considerations, realistic performance expectations, and application to reverse engineering of vaccines for AIDS. J. Med. Primatol. 34:303–312 10.1111/j.1600-0684.2005.00128.x - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

