Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo
- PMID: 22451716
- PMCID: PMC3328366
- DOI: 10.1084/jem.20112322
Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo
Abstract
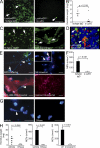
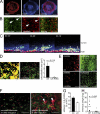
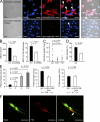
Deep vein thrombosis (DVT) is a major cause of cardiovascular death. The sequence of events that promote DVT remains obscure, largely as a result of the lack of an appropriate rodent model. We describe a novel mouse model of DVT which reproduces a frequent trigger and resembles the time course, histological features, and clinical presentation of DVT in humans. We demonstrate by intravital two-photon and epifluorescence microscopy that blood monocytes and neutrophils crawling along and adhering to the venous endothelium provide the initiating stimulus for DVT development. Using conditional mutants and bone marrow chimeras, we show that intravascular activation of the extrinsic pathway of coagulation via tissue factor (TF) derived from myeloid leukocytes causes the extensive intraluminal fibrin formation characteristic of DVT. We demonstrate that thrombus-resident neutrophils are indispensable for subsequent DVT propagation by binding factor XII (FXII) and by supporting its activation through the release of neutrophil extracellular traps (NETs). Correspondingly, neutropenia, genetic ablation of FXII, or disintegration of NETs each confers protection against DVT amplification. Platelets associate with innate immune cells via glycoprotein Ibα and contribute to DVT progression by promoting leukocyte recruitment and stimulating neutrophil-dependent coagulation. Hence, we identified a cross talk between monocytes, neutrophils, and platelets responsible for the initiation and amplification of DVT and for inducing its unique clinical features.
Figures

Similar articles
-
Neutrophil extracellular trap (NET) impact on deep vein thrombosis.Arterioscler Thromb Vasc Biol. 2012 Aug;32(8):1777-83. doi: 10.1161/ATVBAHA.111.242859. Epub 2012 May 31. Arterioscler Thromb Vasc Biol. 2012. PMID: 22652600 Free PMC article. Review.
-
Tissue factor expressed by circulating cancer cell-derived microparticles drastically increases the incidence of deep vein thrombosis in mice.J Thromb Haemost. 2015 Jul;13(7):1310-9. doi: 10.1111/jth.13002. Epub 2015 Jun 8. J Thromb Haemost. 2015. PMID: 25955268 Free PMC article.
-
Tissue factor-positive neutrophils bind to injured endothelial wall and initiate thrombus formation.Blood. 2012 Sep 6;120(10):2133-43. doi: 10.1182/blood-2012-06-437772. Epub 2012 Jul 26. Blood. 2012. PMID: 22837532
-
Activated αIIbβ3 on platelets mediates flow-dependent NETosis via SLC44A2.Elife. 2020 Apr 21;9:e53353. doi: 10.7554/eLife.53353. Elife. 2020. PMID: 32314961 Free PMC article.
-
Crossroads of coagulation and innate immunity: the case of deep vein thrombosis.J Thromb Haemost. 2013 Jun;11 Suppl 1:233-41. doi: 10.1111/jth.12261. J Thromb Haemost. 2013. PMID: 23809127 Review.
Cited by
-
Historical lessons in translational medicine: cyclooxygenase inhibition and P2Y12 antagonism.Circ Res. 2013 Jan 4;112(1):174-94. doi: 10.1161/CIRCRESAHA.111.300271. Circ Res. 2013. PMID: 23287454 Free PMC article.
-
Diverse novel functions of neutrophils in immunity, inflammation, and beyond.J Exp Med. 2013 Jul 1;210(7):1283-99. doi: 10.1084/jem.20122220. J Exp Med. 2013. PMID: 23825232 Free PMC article. Review.
-
Ginsenoside Rg5 allosterically interacts with P2RY12 and ameliorates deep venous thrombosis by counteracting neutrophil NETosis and inflammatory response.Front Immunol. 2022 Aug 12;13:918476. doi: 10.3389/fimmu.2022.918476. eCollection 2022. Front Immunol. 2022. PMID: 36032109 Free PMC article.
-
Oxidative stress-induced fibrinogen modifications in liver transplant recipients: unraveling a novel potential mechanism for cardiovascular risk.Res Pract Thromb Haemost. 2024 Aug 23;8(6):102555. doi: 10.1016/j.rpth.2024.102555. eCollection 2024 Aug. Res Pract Thromb Haemost. 2024. PMID: 39309232 Free PMC article.
-
Platelet in thrombo-inflammation: Unraveling new therapeutic targets.Front Immunol. 2022 Nov 14;13:1039843. doi: 10.3389/fimmu.2022.1039843. eCollection 2022. Front Immunol. 2022. PMID: 36451834 Free PMC article. Review.
References
-
- Brill A., Fuchs T.A., Chauhan A.K., Yang J.J., De Meyer S.F., Köllnberger M., Wakefield T.W., Lämmle B., Massberg S., Wagner D.D. 2011. von Willebrand factor-mediated platelet adhesion is critical for deep vein thrombosis in mouse models. Blood. 117:1400–1407 10.1182/blood-2010-05-287623 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

