CD80 expression on B cells regulates murine T follicular helper development, germinal center B cell survival, and plasma cell generation
- PMID: 22450810
- PMCID: PMC3331930
- DOI: 10.4049/jimmunol.1102885
CD80 expression on B cells regulates murine T follicular helper development, germinal center B cell survival, and plasma cell generation
Abstract
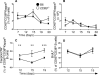
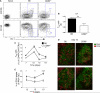
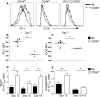
Germinal center (GC) B cells and T follicular helper (T(FH)) cells interact in the production of high-affinity long-lived plasma cells (PCs) and memory B cells, although the mechanisms regulating the formation of these long-lived populations remain unclear. Because CD80 is one of the few markers shared by human and murine memory B cells, we investigated its role in the development of GCs, memory cells, and PCs. In CD80-deficient mice, fewer long-lived PCs were generated upon immunization compared with that in B6 controls. In concert, the absence of CD80 resulted in an increase in apoptotic GC B cells during the contraction phase of the GC. CD80(-/-) mice had fewer T(FH) cells compared with that of B6, and residual T(FH) cells failed to mature, with decreased ICOS and PD-1 expression and decreased synthesis of IL-21 mRNA. Mixed bone marrow chimeras demonstrated a B cell-intrinsic requirement for CD80 expression for normal T(FH) cell and PC development. Therefore, B cell expression of CD80 plays a critical role in regulating B-T interactions in both early and late GC responses. This, in turn, results in impaired ability to produce long-lived PCs. These data provide new insights into the development of GCs and Ab-forming cells and the functions of CD80 in humoral immunity.
Figures
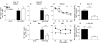
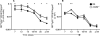
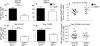
Similar articles
-
Transcription factor IRF4 determines germinal center formation through follicular T-helper cell differentiation.Proc Natl Acad Sci U S A. 2012 May 29;109(22):8664-9. doi: 10.1073/pnas.1205834109. Epub 2012 May 2. Proc Natl Acad Sci U S A. 2012. PMID: 22552227 Free PMC article.
-
The role of ICOS in the CXCR5+ follicular B helper T cell maintenance in vivo.J Immunol. 2005 Aug 15;175(4):2340-8. doi: 10.4049/jimmunol.175.4.2340. J Immunol. 2005. PMID: 16081804
-
B7-H1 expression on non-B and non-T cells promotes distinct effects on T- and B-cell responses in autoimmune arthritis.Eur J Immunol. 2010 Nov;40(11):3117-27. doi: 10.1002/eji.201040690. Epub 2010 Oct 27. Eur J Immunol. 2010. PMID: 21061440 Free PMC article.
-
Development and function of follicular helper T cells.Biosci Biotechnol Biochem. 2016;80(1):1-6. doi: 10.1080/09168451.2015.1056512. Epub 2015 Jun 29. Biosci Biotechnol Biochem. 2016. PMID: 26120879 Review.
-
Germinal center B and follicular helper T cells: siblings, cousins or just good friends?Nat Immunol. 2011 Jun;12(6):472-7. doi: 10.1038/ni.2019. Nat Immunol. 2011. PMID: 21739669 Review.
Cited by
-
The CD28 Transmembrane Domain Contains an Essential Dimerization Motif.Front Immunol. 2020 Jul 16;11:1519. doi: 10.3389/fimmu.2020.01519. eCollection 2020. Front Immunol. 2020. PMID: 32765524 Free PMC article.
-
Innate and adaptive signals enhance differentiation and expansion of dual-antibody autoreactive B cells in lupus.Nat Commun. 2018 Sep 28;9(1):3973. doi: 10.1038/s41467-018-06293-z. Nat Commun. 2018. PMID: 30266981 Free PMC article.
-
T cell receptor signaling can directly enhance the avidity of CD28 ligand binding.PLoS One. 2014 Feb 24;9(2):e89263. doi: 10.1371/journal.pone.0089263. eCollection 2014. PLoS One. 2014. PMID: 24586641 Free PMC article.
-
A Temporal Switch in the Germinal Center Determines Differential Output of Memory B and Plasma Cells.Immunity. 2016 Jan 19;44(1):116-130. doi: 10.1016/j.immuni.2015.12.004. Epub 2016 Jan 12. Immunity. 2016. PMID: 26795247 Free PMC article.
-
CD73 expression identifies a subset of IgM+ antigen-experienced cells with memory attributes that is T cell and CD40 signalling dependent.Immunology. 2017 Dec;152(4):602-612. doi: 10.1111/imm.12800. Epub 2017 Aug 23. Immunology. 2017. PMID: 28746783 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

