Ablation of the pro-apoptotic protein Bax protects mice from glucocorticoid-induced bone growth impairment
- PMID: 22442678
- PMCID: PMC3307731
- DOI: 10.1371/journal.pone.0033168
Ablation of the pro-apoptotic protein Bax protects mice from glucocorticoid-induced bone growth impairment
Abstract
Dexamethasone (Dexa) is a widely used glucocorticoid to treat inflammatory diseases; however, a multitude of undesired effects have been reported to arise from this treatment including osteoporosis, obesity, and in children decreased longitudinal bone growth. We and others have previously shown that glucocorticoids induce apoptosis in growth plate chondrocytes. Here, we hypothesized that Bax, a pro-apoptotic member of the Bcl-2 family, plays a key role in Dexa-induced chondrocyte apoptosis and bone growth impairment. Indeed, experiments in the human HCS-2/8 chondrocytic cell line demonstrated that silencing of Bax expression using small-interfering (si) RNA efficiently blocked Dexa-induced apoptosis. Furthermore, ablation of Bax in female mice protected against Dexa-induced bone growth impairment. Finally, Bax activation by Dexa was confirmed in human growth plate cartilage specimens cultured ex vivo. Our findings could therefore open the door for new therapeutic approaches to prevent glucocorticoid-induced bone growth impairment through specific targeting of Bax.
Conflict of interest statement
Figures
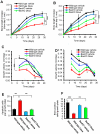
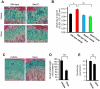
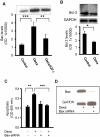
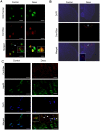
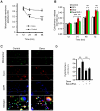
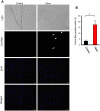
Comment in
-
Bone: Safer glucocorticoid therapy--Bax to the future?Nat Rev Rheumatol. 2012 Apr 10;8(5):248. doi: 10.1038/nrrheum.2012.55. Nat Rev Rheumatol. 2012. PMID: 22487800 No abstract available.
Similar articles
-
Bortezomib is cytotoxic to the human growth plate and permanently impairs bone growth in young mice.PLoS One. 2012;7(11):e50523. doi: 10.1371/journal.pone.0050523. Epub 2012 Nov 30. PLoS One. 2012. PMID: 23226303 Free PMC article.
-
Dexamethasone differentially regulates Bcl-2 family proteins in human proliferative chondrocytes: role of pro-apoptotic Bid.Toxicol Lett. 2014 Jan 13;224(2):196-200. doi: 10.1016/j.toxlet.2013.10.020. Epub 2013 Oct 27. Toxicol Lett. 2014. PMID: 24172751
-
Dexamethasone induces apoptosis in proliferative chondrocytes through activation of caspases and suppression of the Akt-phosphatidylinositol 3'-kinase signaling pathway.Endocrinology. 2005 Mar;146(3):1391-7. doi: 10.1210/en.2004-1152. Epub 2004 Dec 2. Endocrinology. 2005. PMID: 15576458
-
Humanin prevents undesired apoptosis of chondrocytes without interfering with the anti-inflammatory effect of dexamethasone in collagen-induced arthritis.Clin Exp Rheumatol. 2020 Jan-Feb;38(1):129-135. Epub 2019 Jun 6. Clin Exp Rheumatol. 2020. PMID: 31172921
-
Growth retardation induced by dexamethasone is associated with increased apoptosis of the growth plate chondrocytes.J Endocrinol. 2003 Mar;176(3):331-7. doi: 10.1677/joe.0.1760331. J Endocrinol. 2003. PMID: 12630918
Cited by
-
Bortezomib is cytotoxic to the human growth plate and permanently impairs bone growth in young mice.PLoS One. 2012;7(11):e50523. doi: 10.1371/journal.pone.0050523. Epub 2012 Nov 30. PLoS One. 2012. PMID: 23226303 Free PMC article.
-
TNF overexpression and dexamethasone treatment impair chondrogenesis and bone growth in an additive manner.Sci Rep. 2022 Oct 28;12(1):18189. doi: 10.1038/s41598-022-22734-8. Sci Rep. 2022. PMID: 36307458 Free PMC article.
-
Dietary 2-oxoglutarate prevents bone loss caused by neonatal treatment with maximal dexamethasone dose.Exp Biol Med (Maywood). 2017 Apr;242(7):671-682. doi: 10.1177/1535370217693322. Epub 2017 Jan 1. Exp Biol Med (Maywood). 2017. PMID: 28178857 Free PMC article.
-
Humanin is a novel regulator of Hedgehog signaling and prevents glucocorticoid-induced bone growth impairment.FASEB J. 2019 Apr;33(4):4962-4974. doi: 10.1096/fj.201801741R. Epub 2019 Jan 18. FASEB J. 2019. PMID: 30657335 Free PMC article.
-
Lithium rescues cultured rat metatarsals from dexamethasone-induced growth failure.Pediatr Res. 2024 Sep;96(4):952-963. doi: 10.1038/s41390-024-03192-6. Epub 2024 Apr 29. Pediatr Res. 2024. PMID: 38684886 Free PMC article.
References
-
- Yeh TF, Lin YJ, Lin HC, Huang CC, Hsieh WS, et al. Outcomes at school age after postnatal dexamethasone therapy for lung disease of prematurity. N Engl J Med. 2004;350:1304–1313. - PubMed
-
- Smink JJ, Gresnigt MG, Hamers N, Koedam JA, Berger R, et al. Short-term glucocorticoid treatment of prepubertal mice decreases growth and IGF-I expression in the growth plate. J Endocrinol. 2003;177:381–388. - PubMed
-
- Mushtaq T, Bijman P, Ahmed SF, Farquharson C. Insulin-like growth factor-I augments chondrocyte hypertrophy and reverses glucocorticoid-mediated growth retardation in fetal mice metatarsal cultures. Endocrinology. 2004;145:2478–2486. - PubMed
-
- Chrysis D, Zaman F, Chagin AS, Takigawa M, Savendahl L. Dexamethasone induces apoptosis in proliferative chondrocytes through activation of caspases and suppression of the Akt-phosphatidylinositol 3′-kinase signaling pathway. Endocrinology. 2005;146:1391–1397. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

