Type I interferons: diversity of sources, production pathways and effects on immune responses
- PMID: 22440910
- PMCID: PMC3572907
- DOI: 10.1016/j.coviro.2011.10.026
Type I interferons: diversity of sources, production pathways and effects on immune responses
Abstract
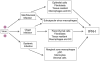
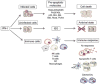
Type I interferons (IFN-I) were first described over 50 years ago as factors produced by cells that interfere with virus replication and promote an antiviral state. Innate and adaptive immune responses to viruses are also greatly influenced by IFN-I. In this article we discuss the diversity of cellular sources of IFN-I and the pathways leading to IFN-I production during viral infections. Finally, we discuss the effects of IFN-I on cells of the immune system with emphasis on dendritic cells.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures


Similar articles
-
Cytosolic surveillance and antiviral immunity.Curr Opin Virol. 2011 Dec;1(6):455-62. doi: 10.1016/j.coviro.2011.11.004. Epub 2011 Dec 4. Curr Opin Virol. 2011. PMID: 22440909 Free PMC article. Review.
-
Balancing viral replication in spleen and liver determines the outcome of systemic virus infection.Z Gastroenterol. 2015 Dec;53(12):1432-5. doi: 10.1055/s-0041-109631. Epub 2015 Dec 14. Z Gastroenterol. 2015. PMID: 26666281 Review.
-
Innate immune response to viral infection.Cytokine. 2008 Sep;43(3):336-41. doi: 10.1016/j.cyto.2008.07.009. Epub 2008 Aug 9. Cytokine. 2008. PMID: 18694646 Review.
-
Innate barriers to viral infection.Future Microbiol. 2012 Jul;7(7):815-22. doi: 10.2217/fmb.12.52. Future Microbiol. 2012. PMID: 22827304
-
Exposing viruses: RNA patterns sensed by RIG-I-like receptors.J Clin Immunol. 2010 Jul;30(4):491-5. doi: 10.1007/s10875-010-9384-7. Epub 2010 Mar 31. J Clin Immunol. 2010. PMID: 20354786 Review.
Cited by
-
A hybrid discrete-continuum model of immune responses to SARS-CoV-2 infection in the lung alveolar region, with a focus on interferon induced innate response.J Theor Biol. 2022 Dec 21;555:111293. doi: 10.1016/j.jtbi.2022.111293. Epub 2022 Oct 5. J Theor Biol. 2022. PMID: 36208668 Free PMC article.
-
ITGB4 Deficiency in Airway Epithelium Aggravates RSV Infection and Increases HDM Sensitivity.Front Immunol. 2022 Jul 25;13:912095. doi: 10.3389/fimmu.2022.912095. eCollection 2022. Front Immunol. 2022. PMID: 35958591 Free PMC article.
-
HIV-Host Cell Interactions.Cells. 2023 May 9;12(10):1351. doi: 10.3390/cells12101351. Cells. 2023. PMID: 37408185 Free PMC article. Review.
-
Plasmacytoid dendritic cells are productively infected and activated through TLR-7 early after arenavirus infection.Cell Host Microbe. 2012 Jun 14;11(6):617-30. doi: 10.1016/j.chom.2012.04.017. Cell Host Microbe. 2012. PMID: 22704622 Free PMC article.
-
Immunogenic cell death triggered by impaired deubiquitination in multiple myeloma relies on dysregulated type I interferon signaling.Front Immunol. 2023 Mar 2;14:982720. doi: 10.3389/fimmu.2023.982720. eCollection 2023. Front Immunol. 2023. PMID: 36936919 Free PMC article.
References
-
- Nagano Y, Kojima Y. Immunizing property of vaccinia virus inactivated by ultraviolets rays. C R Seances Soc Biol Fil. 1954;148:1700–1702. - PubMed
-
- Pestka S, Krause CD, Walter MR. Interferons, interferon-like cytokines, and their receptors. Immunol Rev. 2004;202:8–32. - PubMed
-
- Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat Immunol. 2004;5:1219–1226. - PubMed
-
- Gilliet M, Cao W, Liu YJ. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat Rev Immunol. 2008;8:594–606. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

