Virus factories, double membrane vesicles and viroplasm generated in animal cells
- PMID: 22440839
- PMCID: PMC7102809
- DOI: 10.1016/j.coviro.2011.09.008
Virus factories, double membrane vesicles and viroplasm generated in animal cells
Abstract
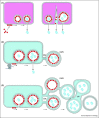
Many viruses reorganise cellular membrane compartments and the cytoskeleton to generate subcellular microenvironments called virus factories or 'viroplasm'. These create a platform to concentrate replicase proteins, virus genomes and host proteins required for replication and also protect against antiviral defences. There is growing interest in understanding how viruses induce such large changes in cellular organisation, and recent studies are beginning to reveal the relationship between virus factories and viroplasm and the cellular structures that house them. In this review, we discuss how three supergroups of (+)RNA viruses generate replication sites from membrane-bound organelles and highlight research on perinuclear factories induced by the nucleocytoplasmic large DNA viruses.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures

Similar articles
-
A guide to viral inclusions, membrane rearrangements, factories, and viroplasm produced during virus replication.Adv Virus Res. 2007;70:101-82. doi: 10.1016/S0065-3527(07)70004-0. Adv Virus Res. 2007. PMID: 17765705 Free PMC article. Review.
-
Aggresomes and autophagy generate sites for virus replication.Science. 2006 May 12;312(5775):875-8. doi: 10.1126/science.1126766. Science. 2006. PMID: 16690857 Review.
-
Virus factories: associations of cell organelles for viral replication and morphogenesis.Biol Cell. 2005 Feb;97(2):147-72. doi: 10.1042/BC20040058. Biol Cell. 2005. PMID: 15656780 Free PMC article. Review.
-
DNA virus replication compartments.J Virol. 2014 Feb;88(3):1404-20. doi: 10.1128/JVI.02046-13. Epub 2013 Nov 20. J Virol. 2014. PMID: 24257611 Free PMC article. Review.
-
Ultrastructure of the replication sites of positive-strand RNA viruses.Virology. 2015 May;479-480:418-33. doi: 10.1016/j.virol.2015.02.029. Epub 2015 Mar 6. Virology. 2015. PMID: 25746936 Free PMC article. Review.
Cited by
-
From Mimivirus to Mirusvirus: The Quest for Hidden Giants.Viruses. 2023 Aug 17;15(8):1758. doi: 10.3390/v15081758. Viruses. 2023. PMID: 37632100 Free PMC article. Review.
-
Severe acute respiratory syndrome coronavirus nonstructural proteins 3, 4, and 6 induce double-membrane vesicles.mBio. 2013 Aug 13;4(4):e00524-13. doi: 10.1128/mBio.00524-13. mBio. 2013. PMID: 23943763 Free PMC article.
-
A Student's Guide to Giant Viruses Infecting Small Eukaryotes: From Acanthamoeba to Zooxanthellae.Viruses. 2017 Mar 17;9(3):46. doi: 10.3390/v9030046. Viruses. 2017. PMID: 28304329 Free PMC article. Review.
-
Filling Knowledge Gaps for Mimivirus Entry, Uncoating, and Morphogenesis.J Virol. 2017 Oct 27;91(22):e01335-17. doi: 10.1128/JVI.01335-17. Print 2017 Nov 15. J Virol. 2017. PMID: 28878069 Free PMC article.
-
Negri bodies are viral factories with properties of liquid organelles.Nat Commun. 2017 Jul 5;8(1):58. doi: 10.1038/s41467-017-00102-9. Nat Commun. 2017. PMID: 28680096 Free PMC article.
References
-
- Cottam E., Pierini R., Roberts R., Wileman T. Origins of membrane vesicles generated during replication of positive-strand RNA viruses. Future Virol. 2009;4:473–485.
-
- den Boon J.A., Ahlquist P. Organelle-like membrane compartmentalization of positive-strand RNA virus replication factories. Annu Rev Microbiol. 2010;64:241–256. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- BB/F012861/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/I/00001439/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/F012861/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/E018521/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/E018521/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Medical

