Improving matrix metalloproteinase-2 specific response of a hydrogel system using electrophoresis
- PMID: 22440150
- PMCID: PMC3327771
- DOI: 10.1016/j.ijpharm.2012.03.012
Improving matrix metalloproteinase-2 specific response of a hydrogel system using electrophoresis
Abstract
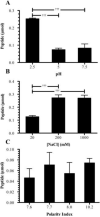
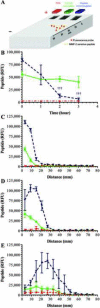
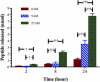
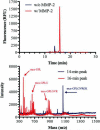
Matrix metalloproteinases (MMPs) overexpression plays a critical role in cancer invasion and metastasis. We utilized this key feature of tumor microenvironment to develop a disease-stimuli triggered drug delivery system. Poly(acrylic acid) hydrogels were synthesized by UV polymerization and pendant MMP-2 sensitive peptides (Gly-Pro-Leu-Gly-Val-Arg-Gly-Lys) conjugated throughout using EDC/sulfo-NHS chemistry. There were significantly more peptides released in the presence of MMP-2 compared with the control groups. The released peptide fragments were analyzed by HPLC and MALDI-MS and confirmed to be the expected fragments. In order to avoid nonspecific release of nonconjugated (i.e. unreacted) peptides, a novel method of electrophoretic washing was developed disrupting the strong electrostatic interactions between the peptides and the pendant groups of the hydrogel. After electrophoresis, the nonspecific peptide release in the absence of MMP-2 was minimized. This newly developed purification system significantly improved the control of release to be in response of the magnitude of the stimuli, i.e. MMP. Specifically, peptides were released proportionally to the concentration of MMP-2 present. Now that many of the design parameters have been examined, anticancer drugs will be conjugated to the MMP sensitive peptide linkers with the goal of implantation in a tumor void releasing anticancer reagent in response to elevated level of MMPs.
Copyright © 2012 Elsevier B.V. All rights reserved.
Figures
Similar articles
-
Matrix metalloprotease selective peptide substrates cleavage within hydrogel matrices for cancer chemotherapy activation.Peptides. 2008 Nov;29(11):1965-73. doi: 10.1016/j.peptides.2008.06.021. Epub 2008 Jul 5. Peptides. 2008. PMID: 18652863 Free PMC article.
-
Effects of molecular weight and loading on matrix metalloproteinase-2 mediated release from poly(ethylene glycol) diacrylate hydrogels.AAPS J. 2012 Sep;14(3):482-90. doi: 10.1208/s12248-012-9356-3. Epub 2012 Apr 26. AAPS J. 2012. PMID: 22535508 Free PMC article.
-
Matrix metalloproteinases-2/9-sensitive peptide-conjugated polymer micelles for site-specific release of drugs and enhancing tumor accumulation: preparation and in vitro and in vivo evaluation.Int J Nanomedicine. 2016 Apr 22;11:1643-61. doi: 10.2147/IJN.S101030. eCollection 2016. Int J Nanomedicine. 2016. PMID: 27217744 Free PMC article.
-
A review of matrix metalloproteinase-2-sensitive nanoparticles as a novel drug delivery for tumor therapy.Int J Biol Macromol. 2024 Mar;262(Pt 2):130043. doi: 10.1016/j.ijbiomac.2024.130043. Epub 2024 Feb 8. Int J Biol Macromol. 2024. PMID: 38340921 Review.
-
Matrix Metalloproteinases (MMPs) in Targeted Drug Delivery: Synthesis of a Potent and Highly Selective Inhibitor against Matrix Metalloproteinase- 7.Curr Top Med Chem. 2020;20(27):2459-2471. doi: 10.2174/1568026620666200722104928. Curr Top Med Chem. 2020. PMID: 32703131 Review.
Cited by
-
Matrix-metalloproteinases as targets for controlled delivery in cancer: An analysis of upregulation and expression.J Control Release. 2017 Aug 10;259:62-75. doi: 10.1016/j.jconrel.2017.01.034. Epub 2017 Jan 31. J Control Release. 2017. PMID: 28153760 Free PMC article. Review.
-
In-house preparation of hydrogels for batch affinity purification of glutathione S-transferase tagged recombinant proteins.BMC Biotechnol. 2012 Sep 18;12:63. doi: 10.1186/1472-6750-12-63. BMC Biotechnol. 2012. PMID: 22989306 Free PMC article.
-
Activated Charge-Reversal Polymeric Nano-System: The Promising Strategy in Drug Delivery for Cancer Therapy.Polymers (Basel). 2016 Apr 5;8(4):99. doi: 10.3390/polym8040099. Polymers (Basel). 2016. PMID: 30979214 Free PMC article. Review.
-
Arginine-rich, cell penetrating peptide-anti-microRNA complexes decrease glioblastoma migration potential.Peptides. 2014 Aug;58:83-90. doi: 10.1016/j.peptides.2014.06.008. Epub 2014 Jun 23. Peptides. 2014. PMID: 24969623 Free PMC article.
-
Proteolytically activated anti-bacterial hydrogel microspheres.J Control Release. 2013 Nov 10;171(3):288-95. doi: 10.1016/j.jconrel.2013.06.023. Epub 2013 Jun 28. J Control Release. 2013. PMID: 23816641 Free PMC article.
References
-
- Bae M, Cho S, Song J, Lee GY, Kim K, Yang J, Cho K, Kim SY, Byun Y. Metalloprotease-specific poly(ethylene glycol) methyl ether-peptide-doxorubicin conjugate for targeting anticancer drug delivery based on angiogenesis. Drugs Exptl. Clin. Res. 2003;29:15–23. - PubMed
-
- Braithwaite A, Smith FJ, Stock R. Chromatographic methods. 4th ed. Chapman and Hall; London; New York: 1985.
-
- Brandl F, Hammer N, Blunk T, Tessmar J, Goepferich A. Biodegradable Hydrogels for Time-Controlled Release of Tethered Peptides or Proteins. Biomacromolecules. 2010;11:496–504. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS055095/NS/NINDS NIH HHS/United States
- R01 NS055095-01A2/NS/NINDS NIH HHS/United States
- C06 RR015482/RR/NCRR NIH HHS/United States
- R01 NS055095-02/NS/NINDS NIH HHS/United States
- R03 EY014357-01/EY/NEI NIH HHS/United States
- R01 NS055095-03/NS/NINDS NIH HHS/United States
- R03 EY014357-02/EY/NEI NIH HHS/United States
- C06 RR015482-01/RR/NCRR NIH HHS/United States
- R01 NS055095-03S1/NS/NINDS NIH HHS/United States
- R01 NS055095-05/NS/NINDS NIH HHS/United States
- C06 RR15482/RR/NCRR NIH HHS/United States
- R03 EY014357-03/EY/NEI NIH HHS/United States
- R03 EY014357/EY/NEI NIH HHS/United States
- R01 NS055095-04/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous

