Regulation of NF-κB by deubiquitinases
- PMID: 22435550
- PMCID: PMC3540820
- DOI: 10.1111/j.1600-065X.2012.01100.x
Regulation of NF-κB by deubiquitinases
Abstract
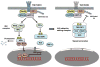
The nuclear factor-κB (NF-κB) pathway is a critical regulator of innate and adaptive immunity. Noncanonical K63-linked polyubiquitination plays a key regulatory role in NF-κB signaling pathways by functioning as a scaffold to recruit kinase complexes containing ubiquitin-binding domains. Ubiquitination is balanced by deubiquitinases that cleave polyubiquitin chains and oppose the function of E3 ubiquitin ligases. Deubiquitinases therefore play an important role in the termination of NF-κB signaling and the resolution of inflammation. In this review, we focus on NF-κB regulation by deubiquitinases with an emphasis on A20 and CYLD. Deubiquitinases and the ubiquitin/proteasome components that regulate NF-κB may serve as novel therapeutic targets for inflammatory diseases and cancer.
© 2012 John Wiley & Sons A/S.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Deubiquitinases in the regulation of NF-κB signaling.Cell Res. 2011 Jan;21(1):22-39. doi: 10.1038/cr.2010.166. Epub 2010 Nov 30. Cell Res. 2011. PMID: 21119682 Free PMC article. Review.
-
Regulation of NF-κB signaling by the A20 deubiquitinase.Cell Mol Immunol. 2012 Mar;9(2):123-30. doi: 10.1038/cmi.2011.59. Epub 2012 Feb 20. Cell Mol Immunol. 2012. PMID: 22343828 Free PMC article. Review.
-
Linear ubiquitination-mediated NF-κB regulation and its related disorders.J Biochem. 2013 Oct;154(4):313-23. doi: 10.1093/jb/mvt079. Epub 2013 Aug 21. J Biochem. 2013. PMID: 23969028 Review.
-
Expression, biological activities and mechanisms of action of A20 (TNFAIP3).Biochem Pharmacol. 2010 Dec 15;80(12):2009-20. doi: 10.1016/j.bcp.2010.06.044. Epub 2010 Jul 3. Biochem Pharmacol. 2010. PMID: 20599425 Review.
-
Proinflammatory mediators alter expression of nuclear factor kappa B-regulating deubiquitinases in sinonasal epithelial cells.Int Forum Allergy Rhinol. 2015 Jul;5(7):583-9. doi: 10.1002/alr.21538. Epub 2015 Apr 24. Int Forum Allergy Rhinol. 2015. PMID: 25907801 Free PMC article.
Cited by
-
USP18 negatively regulates NF-κB signaling by targeting TAK1 and NEMO for deubiquitination through distinct mechanisms.Sci Rep. 2015 Aug 4;5:12738. doi: 10.1038/srep12738. Sci Rep. 2015. PMID: 26240016 Free PMC article.
-
NF-κB p65 directs sex-specific neuroprotection in human neurons.Sci Rep. 2018 Oct 30;8(1):16012. doi: 10.1038/s41598-018-34394-8. Sci Rep. 2018. PMID: 30375448 Free PMC article.
-
Loss of Trabid, a new negative regulator of the drosophila immune-deficiency pathway at the level of TAK1, reduces life span.PLoS Genet. 2014 Feb 20;10(2):e1004117. doi: 10.1371/journal.pgen.1004117. eCollection 2014 Feb. PLoS Genet. 2014. PMID: 24586180 Free PMC article.
-
Effects of Taraxerol on Oxidative and Inflammatory Mediators in Isoproterenol-Induced Cardiotoxicity in an Animal Model.Molecules. 2023 May 15;28(10):4089. doi: 10.3390/molecules28104089. Molecules. 2023. PMID: 37241830 Free PMC article.
-
Advances in Deubiquitinating Enzyme Inhibition and Applications in Cancer Therapeutics.Cancers (Basel). 2020 Jun 15;12(6):1579. doi: 10.3390/cancers12061579. Cancers (Basel). 2020. PMID: 32549302 Free PMC article. Review.
References
-
- Sen R, Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986;46:705–16. - PubMed
-
- Vallabhapurapu S, Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693–733. - PubMed
-
- Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18:2195–224. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

