C-MOPP: the forgotten regimen plus Rituximab for untreated and relapsed follicular lymphoma
- PMID: 22432081
- PMCID: PMC3301422
C-MOPP: the forgotten regimen plus Rituximab for untreated and relapsed follicular lymphoma
Abstract
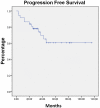
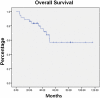
C-MOPP is a chemotherapy regimen for the treatment of Non-Hodgkin lymphoma (NHL). Because rituximab improves results in B-cell NHL, we added rituximab to C-MOPP, giving it the term C-MOPP-R. We retrospectively report the results of C-MOPP-R treatment for follicular lymphoma at Saint Louis University Cancer Center from 2000-2009. Treatment response was assessed with fusion PET/CT using International Harmonization Project Criteria and toxicity using National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0. Thirty-seven patients with follicular lymphoma were treated at our institution with C-MOPP-R. The complete response rate was ninety-four percent and sixty-eight percent in untreated and relapsed patients, respectively. The median progression-free and overall survivals were not reached with median observation time of 34 months. Development of peripheral neuropathy required truncation of planned vincristine dosing in nearly half of patients. We believe that C-MOPP-R results in excellent response rates, progression-free, and overall survival for untreated and relapsed follicular lymphoma and capped vincristine dosing is essential to optimize safety.
Keywords: Lymphoma; Non-Hodgkin; Positron Emission Tomography; antibodies; antineoplastic agents; monoclonal.
Figures
Similar articles
-
Rituximab: a review of its use in chronic lymphocytic leukaemia, low-grade or follicular lymphoma and diffuse large B-cell lymphoma.Drugs. 2010 Jul 30;70(11):1445-76. doi: 10.2165/11201110-000000000-00000. Drugs. 2010. PMID: 20614951 Review.
-
Combination of ibrutinib with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) for treatment-naive patients with CD20-positive B-cell non-Hodgkin lymphoma: a non-randomised, phase 1b study.Lancet Oncol. 2014 Aug;15(9):1019-26. doi: 10.1016/S1470-2045(14)70311-0. Epub 2014 Jul 17. Lancet Oncol. 2014. PMID: 25042202 Clinical Trial.
-
Bortezomib plus rituximab versus rituximab alone in patients with relapsed, rituximab-naive or rituximab-sensitive, follicular lymphoma: a randomised phase 3 trial.Lancet Oncol. 2011 Aug;12(8):773-84. doi: 10.1016/S1470-2045(11)70150-4. Epub 2011 Jul 1. Lancet Oncol. 2011. PMID: 21724462 Clinical Trial.
-
Prognostic value of end-of-induction PET response after first-line immunochemotherapy for follicular lymphoma (GALLIUM): secondary analysis of a randomised, phase 3 trial.Lancet Oncol. 2018 Nov;19(11):1530-1542. doi: 10.1016/S1470-2045(18)30618-1. Epub 2018 Oct 8. Lancet Oncol. 2018. PMID: 30309758
-
Rituximab for the first-line treatment of stage III-IV follicular lymphoma (review of Technology Appraisal No. 110): a systematic review and economic evaluation.Health Technol Assess. 2012;16(37):1-253, iii-iv. doi: 10.3310/hta16370. Health Technol Assess. 2012. PMID: 23021127 Review.
References
-
- Morgenfeld MC, Pavlovsky A, Suarez A, Somoza N, Pavlovsky S, Palau M, Barros CA. Combined cyclophosphamide, vincristine, procarbazine, and prednisone (COPP) therapy of malignant lymphoma. Cancer. 1975;36:1241–1249. - PubMed
-
- Longo DL, Young RC, Hubbard SM, Wesley M, Fisher RI, Jaffe E, Berard C, DeVita VT., Jr Prolonged initial remission in patients with nodular mixed lymphoma. Ann Intern Med. 1984;100:651–656. - PubMed
-
- Ezdinli EZ, Costello WG, Silverstein MN, Berard C, Hartsock RJ, Sokal JE. Moderate versus intensive chemotherapy of prognostically favorable non-Hodgkin's lymphoma. Cancer. 1980;46:29–33. - PubMed
-
- Glick JH, Barnes JM, Ezdinli EZ, Berard CW, Orlow EL, Bennett JM. Nodular mixed lymphoma: results of a randomized trial failing to confirm prolonged disease-free survival with COPP chemotherapy. Blood. 1981;58:920–925. - PubMed
-
- Liang R, Todd D, Chan TK, Chiu E, Lie A, Ho F. COPP chemotherapy for elderly patients with intermediate and high grade non-Hodgkin's lymphoma. Hematol Oncol. 1993;11:43–50. - PubMed
LinkOut - more resources
Full Text Sources