B cells as effectors and regulators of sex-biased arthritis
- PMID: 22429183
- PMCID: PMC3720982
- DOI: 10.3109/08916934.2012.665528
B cells as effectors and regulators of sex-biased arthritis
Abstract
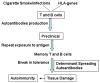
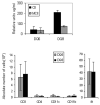
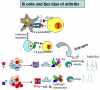
B cells have been implicated both with pathogenic as well as protective capabilities in induction and regulation of autoimmune diseases. Rheumatoid arthritis (RA) is an autoimmune disease that occurs more often in women than men. A significant role of B cells as antibody producing and antigen-presenting cells has been demonstrated in RA. Predisposition to RA is associated with the presence of certain HLA class II alleles that share sequences with DRB1*0401. To determine the role of HLA genes and B cells in vivo, we have generated transgenic mice carrying HLA genes, DRB1*0401 and DQ8, known to be associated with susceptibility to RA. Humanized mice can be induced to develop arthritis that mimics human disease in clinical, histopathological and sex bias. Effect of hormones on immune cells and their function has been described in humans and mice and has been suggested to be the major reason for female bias of autoimmune diseases. An immune response to an antigen requires presentation by HLA molecules thus suggesting a critical role of MHC in combination with sex hormones in susceptibility to develop rheumatoid arthritis. Based on our observations, we hypothesize that modulation of B cells by estrogen, presentation of modified antigens by DR4 and production of antigen-specific B cell modulating cytokines leads to autoreactivity in females. These data suggest that considering patient's sex may be crucial in selecting the optimal treatment strategy. Humanized mice expressing RA susceptible and resistant haplotype provide a means to investigate mechanism sex-bias of arthritis and future strategies for therapy.
Figures

Similar articles
-
To B or not to B: role of B cells in pathogenesis of arthritis in HLA transgenic mice.J Autoimmun. 2011 Sep;37(2):95-103. doi: 10.1016/j.jaut.2011.05.002. Epub 2011 Jun 12. J Autoimmun. 2011. PMID: 21665435 Free PMC article. Review.
-
Mechanism by which HLA-DR4 regulates sex-bias of arthritis in humanized mice.J Autoimmun. 2010 Aug;35(1):1-9. doi: 10.1016/j.jaut.2009.12.007. Epub 2010 Jan 12. J Autoimmun. 2010. PMID: 20061120 Free PMC article.
-
Role of HLA class II genes in susceptibility/resistance to inflammatory arthritis: studies with humanized mice.Immunol Rev. 2010 Jan;233(1):62-78. doi: 10.1111/j.0105-2896.2009.00858.x. Immunol Rev. 2010. PMID: 20192993 Review.
-
New humanized HLA-DR4-transgenic mice that mimic the sex bias of rheumatoid arthritis.Arthritis Rheum. 2007 Jan;56(1):69-78. doi: 10.1002/art.22213. Arthritis Rheum. 2007. PMID: 17195209
-
Induction of arthritis in HLA-DR4-humanized and HLA-DQ8-humanized mice by human cartilage proteoglycan aggrecan but only in the presence of an appropriate (non-MHC) genetic background.Arthritis Rheum. 2004 Jun;50(6):1984-95. doi: 10.1002/art.20285. Arthritis Rheum. 2004. PMID: 15188376
Cited by
-
Sex hormone influence on female-biased autoimmune diseases hints at puberty as an important factor in pathogenesis.Front Pediatr. 2023 Jan 30;11:1051624. doi: 10.3389/fped.2023.1051624. eCollection 2023. Front Pediatr. 2023. PMID: 36793337 Free PMC article. Review.
-
Sex bias in lymphocytes: Implications for autoimmune diseases.Front Immunol. 2022 Nov 24;13:945762. doi: 10.3389/fimmu.2022.945762. eCollection 2022. Front Immunol. 2022. PMID: 36505451 Free PMC article. Review.
-
Sex differences in Tfh cell help to B cells contribute to sexual dimorphism in severity of rat collagen-induced arthritis.Sci Rep. 2020 Jan 27;10(1):1214. doi: 10.1038/s41598-020-58127-y. Sci Rep. 2020. PMID: 31988383 Free PMC article.
-
Mesenchymal stem cells ameliorate B-cell-mediated immune responses and increase IL-10-expressing regulatory B cells in an EBI3-dependent manner.Cell Mol Immunol. 2017 Jan 2;14(11):895-908. doi: 10.1038/cmi.2016.59. Online ahead of print. Cell Mol Immunol. 2017. PMID: 28042143 Free PMC article.
-
Thymic Germinal Centers and Corticosteroids in Myasthenia Gravis: an Immunopathological Study in 1035 Cases and a Critical Review.Clin Rev Allergy Immunol. 2017 Feb;52(1):108-124. doi: 10.1007/s12016-016-8558-3. Clin Rev Allergy Immunol. 2017. PMID: 27273086 Review.
References
-
- Cariappa A, Chase C, Liu H, Russell P, Pillai S. Naive recirculating B cells mature simultaneously in the spleen and bone marrow. Blood. 2007;109:2339–45. - PubMed
-
- Lindsley RC, Thomas M, Srivastava B, Allman D. Generation of peripheral B cells occurs via two spatially and temporally distinct pathways. Blood. 2007;109:2521–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
