Virologic failure of protease inhibitor-based second-line antiretroviral therapy without resistance in a large HIV treatment program in South Africa
- PMID: 22427821
- PMCID: PMC3302781
- DOI: 10.1371/journal.pone.0032144
Virologic failure of protease inhibitor-based second-line antiretroviral therapy without resistance in a large HIV treatment program in South Africa
Abstract
Background: We investigated the prevalence of wild-type virus (no major drug resistance) and drug resistance mutations at second-line antiretroviral treatment (ART) failure in a large HIV treatment program in South Africa.
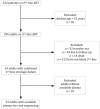
Methodology/ principal findings: HIV-infected patients ≥ 15 years of age who had failed protease inhibitor (PI)-based second-line ART (2 consecutive HIV RNA tests >1000 copies/ml on lopinavir/ritonavir, didanosine, and zidovudine) were identified retrospectively. Patients with virologic failure were continued on second-line ART. Genotypic testing for drug resistance was performed on frozen plasma samples obtained closest to and after the date of laboratory confirmed second-line ART failure. Of 322 HIV-infected patients on second-line ART, 43 were adults with confirmed virologic failure, and 33 had available plasma for viral sequencing. HIV-1 RNA subtype C predominated (n = 32, 97%). Mean duration on ART (SD) prior to initiation of second-line ART was 23 (17) months, and time from second-line ART initiation to failure was 10 (9) months. Plasma samples were obtained 7(9) months from confirmed failure. At second-line failure, 22 patients (67%) had wild-type virus. There was no major resistance to PIs found. Eleven of 33 patients had a second plasma sample taken 8 (5.5) months after the first. Median HIV-1 RNA and the genotypic resistance profile were unchanged.
Conclusions/ significance: Most patients who failed second-line ART had wild-type virus. We did not observe evolution of resistance despite continuation of PI-based ART after failure. Interventions that successfully improve adherence could allow patients to continue to benefit from second-line ART therapy even after initial failure.
Conflict of interest statement
Similar articles
-
Second-line antiretroviral therapy failure and characterization of HIV-1 drug resistance patterns in children in Mali.Arch Pediatr. 2019 Jul;26(5):254-258. doi: 10.1016/j.arcped.2019.06.002. Epub 2019 Jul 12. Arch Pediatr. 2019. PMID: 31307909
-
Study of the impact of HIV genotypic drug resistance testing on therapy efficacy.Verh K Acad Geneeskd Belg. 2001;63(5):447-73. Verh K Acad Geneeskd Belg. 2001. PMID: 11813503 Review.
-
Outcomes after virologic failure of first-line ART in South Africa.AIDS. 2010 Apr 24;24(7):1007-12. doi: 10.1097/QAD.0b013e3283333639. AIDS. 2010. PMID: 20397305 Free PMC article.
-
Intensive adherence counselling for HIV-infected individuals failing second-line antiretroviral therapy in Johannesburg, South Africa.Trop Med Int Health. 2016 Sep;21(9):1131-7. doi: 10.1111/tmi.12741. Epub 2016 Jul 7. Trop Med Int Health. 2016. PMID: 27383454
-
Drug resistance testing through remote genotyping and predicted treatment options in human immunodeficiency virus type 1 infected Tanzanian subjects failing first or second line antiretroviral therapy.PLoS One. 2017 Jun 5;12(6):e0178942. doi: 10.1371/journal.pone.0178942. eCollection 2017. PLoS One. 2017. PMID: 28582463 Free PMC article.
Cited by
-
Prevalence of HIV Drug Resistance Mutations among Treatment-Naive People Living with HIV in a Tertiary Care Center in India.Am J Trop Med Hyg. 2024 Mar 5;110(4):713-718. doi: 10.4269/ajtmh.23-0026. Print 2024 Apr 3. Am J Trop Med Hyg. 2024. PMID: 38442417
-
HIV Drug Resistance Mutations and Subtype Profiles among Pregnant Women of Ho Chi Minh City, South Vietnam.Viruses. 2023 Sep 27;15(10):2008. doi: 10.3390/v15102008. Viruses. 2023. PMID: 37896785 Free PMC article.
-
Second-Line Antiretroviral Treatment Outcomes and Predictors in Tigray Region, Ethiopia.Infect Drug Resist. 2023 Jul 27;16:4903-4912. doi: 10.2147/IDR.S419348. eCollection 2023. Infect Drug Resist. 2023. PMID: 37534062 Free PMC article.
-
Third-line antiretroviral therapy, including raltegravir (RAL), darunavir (DRV/r) and/or etravirine (ETR), is well tolerated and achieves durable virologic suppression over 144 weeks in resource-limited settings: ACTG A5288 strategy trial.J Int AIDS Soc. 2022 Jun;25(6):e25905. doi: 10.1002/jia2.25905. J Int AIDS Soc. 2022. PMID: 36039892 Free PMC article.
-
Rapid HIV-1 drug resistance testing in a resource limited setting: the Pan Degenerate Amplification and Adaptation assay (PANDAA).Pan Afr Med J. 2021 Sep 22;40:57. doi: 10.11604/pamj.2021.40.57.28558. eCollection 2021. Pan Afr Med J. 2021. PMID: 34795836 Free PMC article.
References
-
- UNAIDS Joint United Nations Programme on HIV/AIDS. Country Fact Sheets: South Africa. 2010. Available: http://cfs.unaids.org/factsheet.htm?lng_code=en. Accessed: 2011 June 14.
-
- Long L, Fox M, Sanne I, Rosen S. The high cost of second-line antiretroviral therapy for HIV/AIDS in South Africa. AIDS. 2010;24:915–919. - PubMed
-
- World Health Organization. Prioritizing Second-Line Antiretroviral Drugs for Adults and Adolescents: A Public Health Approach. Geneva: World Health Organization, HIV Department; 2007. Available: http://www.who.int/hiv/pub/meetingreports/Second_Line_Antiretroviral.pdf. Accessed: 2012 February 8.
-
- Walmsley S, Bernstein B, King M, Arribas J, Beall G, et al. Lopinavir-ritonavir versus nelfinavir for the initial treatment of HIV infection. N Engl J Med. 2002;346:2039–2046. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous