Model systems for studying trophoblast differentiation from human pluripotent stem cells
- PMID: 22427062
- PMCID: PMC3429771
- DOI: 10.1007/s00441-012-1371-2
Model systems for studying trophoblast differentiation from human pluripotent stem cells
Abstract
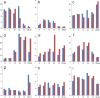
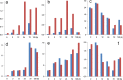
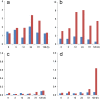
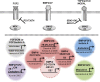
This review focuses on a now well-established model for generating cells of the trophoblast (TB) lineage by treating human embryonic stem cells (ESC) and induced pluripotent stem cells (iPSC) with the growth factor BMP4. We first discuss the opposing roles of FGF2 and BMP4 in directing TB formation and the need to exclude the former from the growth medium to minimize the co-induction of mesoderm and endoderm. Under these conditions, there is up-regulation of several transcription factors implicated in TB lineage emergence within 3 h of BMP4 exposure and, over a period of days and especially under a high O(2) gas atmosphere, gradual appearance of cell types carrying markers for more differentiated TB cell types, including extravillous TB and syncytioTB. We describe the potential value of including low molecular weight pharmaceutical agents that block activin A (INHBA) and FGF2 signaling to support BMP4-directed differentiation. We contend that the weight of available evidence supports the contention that BMP4 converts human ESC and iPSC of the so-called epiblast type unidirectionally to TB. We also consider the argument that BMP4 treatment of human ESC in the absence of exogenous FGF2 leads only to the emergence of mesoderm derivatives to be seriously flawed. Instead, we propose that, when signaling networks supporting pluripotency ESC or iPSC become unsustainable and when specification towards extra-embryonic mesoderm and endoderm are rendered inoperative, TB emerges as a major default state to pluripotency.
Figures

Similar articles
-
Complete and unidirectional conversion of human embryonic stem cells to trophoblast by BMP4.Proc Natl Acad Sci U S A. 2013 Mar 26;110(13):E1212-21. doi: 10.1073/pnas.1303094110. Epub 2013 Mar 14. Proc Natl Acad Sci U S A. 2013. PMID: 23493551 Free PMC article.
-
Heightened potency of human pluripotent stem cell lines created by transient BMP4 exposure.Proc Natl Acad Sci U S A. 2015 May 5;112(18):E2337-46. doi: 10.1073/pnas.1504778112. Epub 2015 Apr 13. Proc Natl Acad Sci U S A. 2015. PMID: 25870291 Free PMC article.
-
Comparison of Four Protocols for In Vitro Differentiation of Human Embryonic Stem Cells into Trophoblast Lineages by BMP4 and Dual Inhibition of Activin/Nodal and FGF2 Signaling.Reprod Sci. 2024 Jan;31(1):173-189. doi: 10.1007/s43032-023-01334-5. Epub 2023 Sep 1. Reprod Sci. 2024. PMID: 37658178 Free PMC article.
-
Specification of trophoblast from embryonic stem cells exposed to BMP4.Biol Reprod. 2018 Jul 1;99(1):212-224. doi: 10.1093/biolre/ioy070. Biol Reprod. 2018. PMID: 29579154 Free PMC article. Review.
-
The role of BMP4 signaling in trophoblast emergence from pluripotency.Cell Mol Life Sci. 2022 Jul 25;79(8):447. doi: 10.1007/s00018-022-04478-w. Cell Mol Life Sci. 2022. PMID: 35877048 Free PMC article. Review.
Cited by
-
Hemochorial placentation: development, function, and adaptations.Biol Reprod. 2018 Jul 1;99(1):196-211. doi: 10.1093/biolre/ioy049. Biol Reprod. 2018. PMID: 29481584 Free PMC article. Review.
-
SMAD1/5 signaling in the early equine placenta regulates trophoblast differentiation and chorionic gonadotropin secretion.Endocrinology. 2014 Aug;155(8):3054-64. doi: 10.1210/en.2013-2116. Epub 2014 May 21. Endocrinology. 2014. PMID: 24848867 Free PMC article.
-
Hypoxia and Placental Development.Birth Defects Res. 2017 Oct 16;109(17):1309-1329. doi: 10.1002/bdr2.1135. Birth Defects Res. 2017. PMID: 29105383 Free PMC article. Review.
-
Tipping the balance toward trophoblast development.Proc Natl Acad Sci U S A. 2016 May 10;113(19):5144-6. doi: 10.1073/pnas.1604914113. Epub 2016 Apr 26. Proc Natl Acad Sci U S A. 2016. PMID: 27118838 Free PMC article. No abstract available.
-
The product of BMP-directed differentiation protocols for human primed pluripotent stem cells is placental trophoblast and not amnion.Stem Cell Reports. 2022 Jun 14;17(6):1289-1302. doi: 10.1016/j.stemcr.2022.04.014. Epub 2022 May 19. Stem Cell Reports. 2022. PMID: 35594861 Free PMC article.
References
-
- Apps R, Murphy SP, Fernando R, Gardner L, Ahad T, Moffett A. Human leucocyte antigen (HLA) expression of primary trophoblast cells and placental cell lines, determined using single antigen beads to characterize allotype specificities of anti-HLA antibodies. Immunology. 2009;127:26–39. doi: 10.1111/j.1365-2567.2008.03019.x. - DOI - PMC - PubMed
-
- Avery S, Zafarana G, Gokhale PJ, Andrews PW. The role of SMAD4 in human embryonic stem cell self-renewal and stem cell fate. Stem Cells. 2010;28:863–873. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

