MicroRNA-214 protects the mouse heart from ischemic injury by controlling Ca²⁺ overload and cell death
- PMID: 22426211
- PMCID: PMC3314458
- DOI: 10.1172/JCI59327
MicroRNA-214 protects the mouse heart from ischemic injury by controlling Ca²⁺ overload and cell death
Abstract
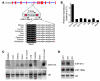
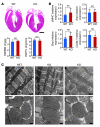
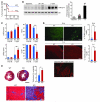
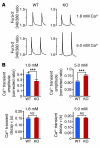
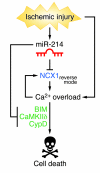
Early reperfusion of ischemic cardiac tissue remains the most effective intervention for improving clinical outcome following myocardial infarction. However, abnormal increases in intracellular Ca²⁺ during myocardial reperfusion can cause cardiomyocyte death and consequent loss of cardiac function, referred to as ischemia/reperfusion (IR) injury. Therapeutic modulation of Ca²⁺ handling provides some cardioprotection against the paradoxical effects of restoring blood flow to the heart, highlighting the significance of Ca²⁺ overload to IR injury. Cardiac IR is also accompanied by dynamic changes in the expression of microRNAs (miRNAs); for example, miR-214 is upregulated during ischemic injury and heart failure, but its potential role in these processes is unknown. Here, we show that genetic deletion of miR-214 in mice causes loss of cardiac contractility, increased apoptosis, and excessive fibrosis in response to IR injury. The cardioprotective roles of miR-214 during IR injury were attributed to repression of the mRNA encoding sodium/calcium exchanger 1 (Ncx1), a key regulator of Ca²⁺ influx; and to repression of several downstream effectors of Ca²⁺ signaling that mediate cell death. These findings reveal a pivotal role for miR-214 as a regulator of cardiomyocyte Ca²⁺ homeostasis and survival during cardiac injury.
Figures
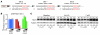


Similar articles
-
MicroRNA-150 protects the mouse heart from ischaemic injury by regulating cell death.Cardiovasc Res. 2015 Jun 1;106(3):387-97. doi: 10.1093/cvr/cvv121. Epub 2015 Mar 30. Cardiovasc Res. 2015. PMID: 25824147 Free PMC article.
-
ROS generated during early reperfusion contribute to intermittent hypobaric hypoxia-afforded cardioprotection against postischemia-induced Ca(2+) overload and contractile dysfunction via the JAK2/STAT3 pathway.J Mol Cell Cardiol. 2015 Apr;81:150-61. doi: 10.1016/j.yjmcc.2015.02.015. Epub 2015 Feb 27. J Mol Cell Cardiol. 2015. PMID: 25731682
-
miR-202-5p protects rat against myocardial ischemia reperfusion injury by downregulating the expression of Trpv2 to attenuate the Ca 2+ overload in cardiomyocytes.J Cell Biochem. 2019 Aug;120(8):13680-13693. doi: 10.1002/jcb.28641. Epub 2019 May 6. J Cell Biochem. 2019. PMID: 31062423
-
Altered Calcium Handling in Reperfusion Injury.Med Chem. 2016;12(2):114-30. doi: 10.2174/1573406411666150928112420. Med Chem. 2016. PMID: 26411605 Review.
-
Mitochondria as a therapeutic target for cardiac ischemia‑reperfusion injury (Review).Int J Mol Med. 2021 Feb;47(2):485-499. doi: 10.3892/ijmm.2020.4823. Epub 2020 Dec 16. Int J Mol Med. 2021. PMID: 33416090 Free PMC article. Review.
Cited by
-
Exosome and its roles in cardiovascular diseases.Heart Fail Rev. 2015 May;20(3):337-48. doi: 10.1007/s10741-014-9469-0. Heart Fail Rev. 2015. PMID: 25549884 Review.
-
Dicer1 activity in the stromal compartment regulates nephron differentiation and vascular patterning during mammalian kidney organogenesis.Kidney Int. 2015 Jun;87(6):1125-40. doi: 10.1038/ki.2014.406. Epub 2015 Feb 4. Kidney Int. 2015. PMID: 25651362 Free PMC article.
-
Knockdown of miR-214 Alleviates Renal Interstitial Fibrosis by Targeting the Regulation of the PTEN/PI3K/AKT Signalling Pathway.Oxid Med Cell Longev. 2022 Oct 15;2022:7553928. doi: 10.1155/2022/7553928. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 36285295 Free PMC article.
-
MicroRNAs in cartilage development and dysplasia.Bone. 2020 Nov;140:115564. doi: 10.1016/j.bone.2020.115564. Epub 2020 Jul 31. Bone. 2020. PMID: 32745689 Free PMC article. Review.
-
Decellularized Extracellular Matrix Hydrogels as a Delivery Platform for MicroRNA and Extracellular Vesicle Therapeutics.Adv Ther (Weinh). 2018 Jul;1(3):1800032. doi: 10.1002/adtp.201800032. Epub 2018 May 28. Adv Ther (Weinh). 2018. PMID: 31544132 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

