Biogenesis of the preprotein translocase of the outer mitochondrial membrane: protein kinase A phosphorylates the precursor of Tom40 and impairs its import
- PMID: 22419819
- PMCID: PMC3338429
- DOI: 10.1091/mbc.E11-11-0933
Biogenesis of the preprotein translocase of the outer mitochondrial membrane: protein kinase A phosphorylates the precursor of Tom40 and impairs its import
Abstract
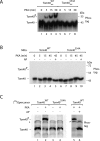
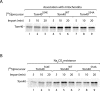
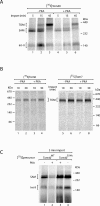
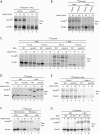
The preprotein translocase of the outer mitochondrial membrane (TOM) functions as the main entry gate for the import of nuclear-encoded proteins into mitochondria. The major subunits of the TOM complex are the three receptors Tom20, Tom22, and Tom70 and the central channel-forming protein Tom40. Cytosolic kinases have been shown to regulate the biogenesis and activity of the Tom receptors. Casein kinase 2 stimulates the biogenesis of Tom22 and Tom20, whereas protein kinase A (PKA) impairs the receptor function of Tom70. Here we report that PKA exerts an inhibitory effect on the biogenesis of the β-barrel protein Tom40. Tom40 is synthesized as precursor on cytosolic ribosomes and subsequently imported into mitochondria. We show that PKA phosphorylates the precursor of Tom40. The phosphorylated Tom40 precursor is impaired in import into mitochondria, whereas the nonphosphorylated precursor is efficiently imported. We conclude that PKA plays a dual role in the regulation of the TOM complex. Phosphorylation by PKA not only impairs the receptor activity of Tom70, but it also inhibits the biogenesis of the channel protein Tom40.
Figures


Similar articles
-
Biogenesis of the mitochondrial TOM complex: Mim1 promotes insertion and assembly of signal-anchored receptors.J Biol Chem. 2008 Jan 4;283(1):120-127. doi: 10.1074/jbc.M706997200. Epub 2007 Nov 1. J Biol Chem. 2008. PMID: 17974559
-
Regulation of mitochondrial protein import by cytosolic kinases.Cell. 2011 Jan 21;144(2):227-39. doi: 10.1016/j.cell.2010.12.015. Epub 2011 Jan 6. Cell. 2011. PMID: 21215441
-
Glucose-induced regulation of protein import receptor Tom22 by cytosolic and mitochondria-bound kinases.Cell Metab. 2013 Oct 1;18(4):578-87. doi: 10.1016/j.cmet.2013.09.006. Cell Metab. 2013. PMID: 24093680
-
Role of the TOM Complex in Protein Import into Mitochondria: Structural Views.Annu Rev Biochem. 2022 Jun 21;91:679-703. doi: 10.1146/annurev-biochem-032620-104527. Epub 2022 Mar 14. Annu Rev Biochem. 2022. PMID: 35287471 Review.
-
How does the TOM complex mediate insertion of precursor proteins into the mitochondrial outer membrane?J Cell Biol. 2005 Nov 7;171(3):419-23. doi: 10.1083/jcb.200507147. Epub 2005 Oct 31. J Cell Biol. 2005. PMID: 16260501 Free PMC article. Review.
Cited by
-
Signaling and Regulation of the Mitochondrial Unfolded Protein Response.Cold Spring Harb Perspect Biol. 2019 Jun 3;11(6):a033944. doi: 10.1101/cshperspect.a033944. Cold Spring Harb Perspect Biol. 2019. PMID: 30617048 Free PMC article. Review.
-
The interaction of the mitochondrial protein importer TOMM34 with HSP70 is regulated by TOMM34 phosphorylation and binding to 14-3-3 adaptors.J Biol Chem. 2020 Jul 3;295(27):8928-8944. doi: 10.1074/jbc.RA120.012624. Epub 2020 May 5. J Biol Chem. 2020. PMID: 32371396 Free PMC article.
-
Cardiac mitochondrial matrix and respiratory complex protein phosphorylation.Am J Physiol Heart Circ Physiol. 2012 Oct 15;303(8):H940-66. doi: 10.1152/ajpheart.00077.2012. Epub 2012 Aug 10. Am J Physiol Heart Circ Physiol. 2012. PMID: 22886415 Free PMC article. Review.
-
After the banquet: mitochondrial biogenesis, mitophagy, and cell survival.Autophagy. 2013 Nov 1;9(11):1663-76. doi: 10.4161/auto.24135. Epub 2013 May 3. Autophagy. 2013. PMID: 23787782 Free PMC article. Review.
-
Common players in mitochondria biogenesis and neuronal protection against stress-induced apoptosis.Neurochem Res. 2014;39(3):546-55. doi: 10.1007/s11064-013-1109-x. Epub 2013 Sep 5. Neurochem Res. 2014. PMID: 24005821 Review.
References
-
- Abe Y, Shodai T, Muto T, Mihara K, Torii H, Nishikawa S, Endo T, Kohda D. Structural basis of presequence recognition by the mitochondrial protein import receptor Tom20. Cell. 2000;100:551–560. - PubMed
-
- Becker L, Bannwarth M, Meisinger C, Hill K, Krimmer T, Casadio R, Truscott KN, Schulz GE, Pfanner N, Wagner R. Preprotein translocase of the outer mitochondrial membrane: reconstituted Tom40 forms a characteristic TOM pore. J Mol Biol. 2005;353:1011–1020. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

