Identification of 4 novel HLA-B*40:01 restricted minor histocompatibility antigens and their potential as targets for graft-versus-leukemia reactivity
- PMID: 22419570
- PMCID: PMC3409817
- DOI: 10.3324/haematol.2011.049478
Identification of 4 novel HLA-B*40:01 restricted minor histocompatibility antigens and their potential as targets for graft-versus-leukemia reactivity
Abstract
Background: Patients with hematologic malignancies can be successfully treated with donor lymphocyte infusion after HLA-matched allogeneic hematopoietic stem cell transplantation. The effect of donor lymphocyte infusion is mediated by donor T cells recognizing minor histocompatibility antigens. T cells recognizing hematopoietic restricted minor histocompatibility antigens may induce selective graft-versus-leukemia reactivity, whereas broadly-expressed antigens may be targeted in graft-versus-host disease.
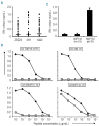
Design and methods: We analyzed in detail CD8(+) T-cell immunity in a patient with relapsed chronic myelogenous leukemia who responded to donor lymphocyte infusion with minimal graft-versus-host disease of the skin. CD8(+) T-cell clones specific for 4 HLA-B*40:01 restricted minor histocompatibility antigens were isolated which were identified by screening a plasmid cDNA library and whole genome association scanning. Detailed T-cell reactivity and monitoring experiments were performed to estimate the clinical and therapeutic relevance of the novel antigens.
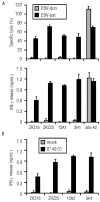
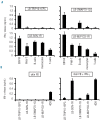
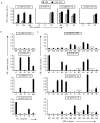
Results: Three antigens were demonstrated to be expressed on primary leukemic cells of various origins as well as subtypes of non-malignant hematopoietic cells, whereas one antigen was selectively recognized on malignant hematopoietic cells with antigen presenting cell phenotype. Skin derived fibroblasts were only recognized after pre-treatment with IFN-γ by two T-cell clones.
Conclusions: Our data show evidence for different roles of the HLA-B*40:01 restricted minor histocompatibility antigens in the onset and execution of the anti-tumor response. All antigens may have contributed to a graft-versus-leukemia effect, and one minor histocompatibility antigen (LB-SWAP70-1Q) has specific therapeutic value based on its in vivo immunodominance and strong presentation on leukemic cells of various origins, but absence of expression on cytokine-treated fibroblasts.
Figures
Similar articles
-
Optimized Whole Genome Association Scanning for Discovery of HLA Class I-Restricted Minor Histocompatibility Antigens.Front Immunol. 2020 Apr 17;11:659. doi: 10.3389/fimmu.2020.00659. eCollection 2020. Front Immunol. 2020. PMID: 32362897 Free PMC article.
-
Identification of 4 new HLA-DR-restricted minor histocompatibility antigens as hematopoietic targets in antitumor immunity.Blood. 2009 Oct 22;114(17):3684-92. doi: 10.1182/blood-2009-03-208017. Epub 2009 Aug 25. Blood. 2009. PMID: 19706888
-
HA-1H T-Cell Receptor Gene Transfer to Redirect Virus-Specific T Cells for Treatment of Hematological Malignancies After Allogeneic Stem Cell Transplantation: A Phase 1 Clinical Study.Front Immunol. 2020 Aug 20;11:1804. doi: 10.3389/fimmu.2020.01804. eCollection 2020. Front Immunol. 2020. PMID: 32973756 Free PMC article. Clinical Trial.
-
Minor histocompatibility antigens to predict, monitor or manipulate GvL and GvHD after allogeneic hematopoietic cell transplantation.Best Pract Res Clin Haematol. 2024 Jun;37(2):101555. doi: 10.1016/j.beha.2024.101555. Epub 2024 May 15. Best Pract Res Clin Haematol. 2024. PMID: 39098803 Review.
-
Minor histocompatibility antigens as targets of graft-versus-leukemia reactions.Curr Opin Hematol. 2002 Nov;9(6):497-502. doi: 10.1097/00062752-200211000-00005. Curr Opin Hematol. 2002. PMID: 12394171 Review.
Cited by
-
Effect of MHC and non-MHC donor/recipient genetic disparity on the outcome of allogeneic HCT.Blood. 2012 Oct 4;120(14):2796-806. doi: 10.1182/blood-2012-04-347286. Epub 2012 Aug 2. Blood. 2012. PMID: 22859606 Free PMC article. Review.
-
The SPPL3-Defined Glycosphingolipid Repertoire Orchestrates HLA Class I-Mediated Immune Responses.Immunity. 2021 Jan 12;54(1):132-150.e9. doi: 10.1016/j.immuni.2020.11.003. Epub 2020 Dec 2. Immunity. 2021. PMID: 33271119 Free PMC article.
-
Microarray Gene Expression Analysis to Evaluate Cell Type Specific Expression of Targets Relevant for Immunotherapy of Hematological Malignancies.PLoS One. 2016 May 12;11(5):e0155165. doi: 10.1371/journal.pone.0155165. eCollection 2016. PLoS One. 2016. PMID: 27171398 Free PMC article.
-
Durable remission of renal cell carcinoma in conjuncture with graft versus host disease following allogeneic stem cell transplantation and donor lymphocyte infusion: rule or exception?PLoS One. 2014 Jan 15;9(1):e85198. doi: 10.1371/journal.pone.0085198. eCollection 2014. PLoS One. 2014. PMID: 24454818 Free PMC article.
-
Whole exome sequencing to estimate alloreactivity potential between donors and recipients in stem cell transplantation.Br J Haematol. 2014 Aug;166(4):566-70. doi: 10.1111/bjh.12898. Epub 2014 Apr 18. Br J Haematol. 2014. PMID: 24749631 Free PMC article.
References
-
- Appelbaum FR. The current status of hematopoietic cell transplantation. Annu Rev Med. 2003;54:491–512. - PubMed
-
- Kolb HJ. Graft-versus-leukemia effects of transplantation and donor lymphocytes. Blood. 2008;112(12):4371–83. - PubMed
-
- Falkenburg JHF, van de Corput L, Marijt WAF, Willemze R. Minor histocompatibility antigens in human stem cell transplantation. Exp Hematol. 2003;31(9):743–51. - PubMed
-
- Mullally A, Ritz J. Beyond HLA: the significance of genomic variation for allogeneic hematopoietic stem cell transplantation. Blood. 2007;109(4):1355–62. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

