Evaluation of cloned cells, animal model, and ATRA sensitivity of human testicular yolk sac tumor
- PMID: 22410253
- PMCID: PMC3314582
- DOI: 10.1186/1479-5876-10-46
Evaluation of cloned cells, animal model, and ATRA sensitivity of human testicular yolk sac tumor
Abstract
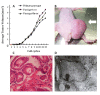
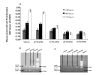
The testicular yolk sac tumor (TYST) is the most common neoplasm originated from germ cells differentiated abnormally, a major part of pediatric malignant testicular tumors. The present study aimed at developing and validating the in vitro and vivo models of TYST and evaluating the sensitivity of TYST to treatments, by cloning human TYST cells and investigating the histology, ultra-structure, growth kinetics and expression of specific proteins of cloned cells. We found biological characteristics of cloned TYST cells were similar to the yolk sac tumor and differentiated from the columnar to glandular-like or goblet cells-like cells. Chromosomes for tumor identification in each passage met nature of the primary tumor. TYST cells were more sensitive to all-trans-retinoic acid which had significantly inhibitory effects on cell proliferation. Cisplatin induced apoptosis of TYST cells through the activation of p53 expression and down-regulation of Bcl- expression. Thus, we believe that cloned TYST cells and the animal model developed here are useful to understand the molecular mechanism of TYST cells and develop potential therapies for human TYST.
© 2012 Zhao et al; licensee BioMed Central Ltd.
Figures




Similar articles
-
Administration of a glypican-3 peptide increases the infiltration and cytotoxicity of CD8+ T cells against testicular yolk sac tumor, associated with enhancing the intratumoral cGAS/STING signaling.Cancer Med. 2023 Dec;12(23):21293-21307. doi: 10.1002/cam4.6605. Epub 2023 Nov 20. Cancer Med. 2023. PMID: 37986544 Free PMC article.
-
Sunitinib inhibits tumor growth and synergizes with cisplatin in orthotopic models of cisplatin-sensitive and cisplatin-resistant human testicular germ cell tumors.Clin Cancer Res. 2009 May 15;15(10):3384-95. doi: 10.1158/1078-0432.CCR-08-2170. Epub 2009 May 5. Clin Cancer Res. 2009. PMID: 19417025
-
[Clinical analysis of stage I pediatric testicular yolk sac tumors: a report of ten cases].Ai Zheng. 2008 Nov;27(11):1226-8. Ai Zheng. 2008. PMID: 19000459 Chinese.
-
Pitfalls in the interpretation of specimens from patients with testicular tumours, with an emphasis on variant morphologies.Pathology. 2018 Jan;50(1):88-99. doi: 10.1016/j.pathol.2017.07.013. Epub 2017 Nov 10. Pathology. 2018. PMID: 29129333 Review.
-
The yolk sac tumor: reflections on a remarkable neoplasm and two of the many intrigued by it-Gunnar Teilum and Aleksander Talerman-and the bond it formed between them.Int J Surg Pathol. 2014 Dec;22(8):677-87. doi: 10.1177/1066896914558265. Epub 2014 Nov 12. Int J Surg Pathol. 2014. PMID: 25395492 Review.
Cited by
-
Recent Advancements in Research on DNA Methylation and Testicular Germ Cell Tumors: Unveiling the Intricate Relationship.Biomedicines. 2024 May 8;12(5):1041. doi: 10.3390/biomedicines12051041. Biomedicines. 2024. PMID: 38791003 Free PMC article. Review.
-
Administration of a glypican-3 peptide increases the infiltration and cytotoxicity of CD8+ T cells against testicular yolk sac tumor, associated with enhancing the intratumoral cGAS/STING signaling.Cancer Med. 2023 Dec;12(23):21293-21307. doi: 10.1002/cam4.6605. Epub 2023 Nov 20. Cancer Med. 2023. PMID: 37986544 Free PMC article.
-
Primary cerebellar endodermal sinus tumor: A case report.Oncol Lett. 2014 Oct;8(4):1713-1716. doi: 10.3892/ol.2014.2340. Epub 2014 Jul 10. Oncol Lett. 2014. PMID: 25202397 Free PMC article.
References
-
- Travis LB, Beard C, Allan JM, Dahl AA, Feldman DR, Oldenburg J, Daugaard G, Kelly JL, Dolan ME, Hannigan R, Constine LS, Oeffinger KC, Okunieff P, Armstrong G, Wiljer D, Miller RC, Gietema JA, van Leeuwen FE, Williams JP, Nichols CR, Einhorn LH, Fossa SD. Testicular cancer survivorship: research strategies and recommendations. J Natl Cancer Inst. 2010;102(15):1114–1130. doi: 10.1093/jnci/djq216. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

